There are minimal data regarding the outcome of patients who have incomplete adherence to DAA therapy or the threshold level of adherence below which the incidence of SVR12 is significantly reduced. Missing doses of DAAs is relatively common. A secondary analysis of data from electronic blister packs that recorded the date and time of each dose among 103 participants in a velpatasvir/sofosbuvir clinical trial demonstrated that approximately one-third (32%) of study participants had <90% adherence (ie, nonadherence) (Cunningham, 2018). The most common episodes of nonadherence lasted 1–2 days (61% of episodes); 11% of episodes lasted ≥7 days. Despite the nonadherence, SVR12 was 94% among both DAA adherent (≥90% of doses) and nonadherent (<90% of doses) patients. Longer durations of missed treatment, however, may affect SVR. A study of patients receiving DAA treatment found that only 50% (2/4) patients with F0-F3 disease who took <4 weeks of their course of therapy experienced SVR, compared with an SVR of 99% (109/110) for those who received ≥4 weeks of therapy. Among patients with cirrhosis, a lower SVR12 rate was observed in those who took <8 weeks of therapy compared with participants who took ≥8 weeks (83% [25/30] vs 95% [209/221]) (Fabbiani, 2021).
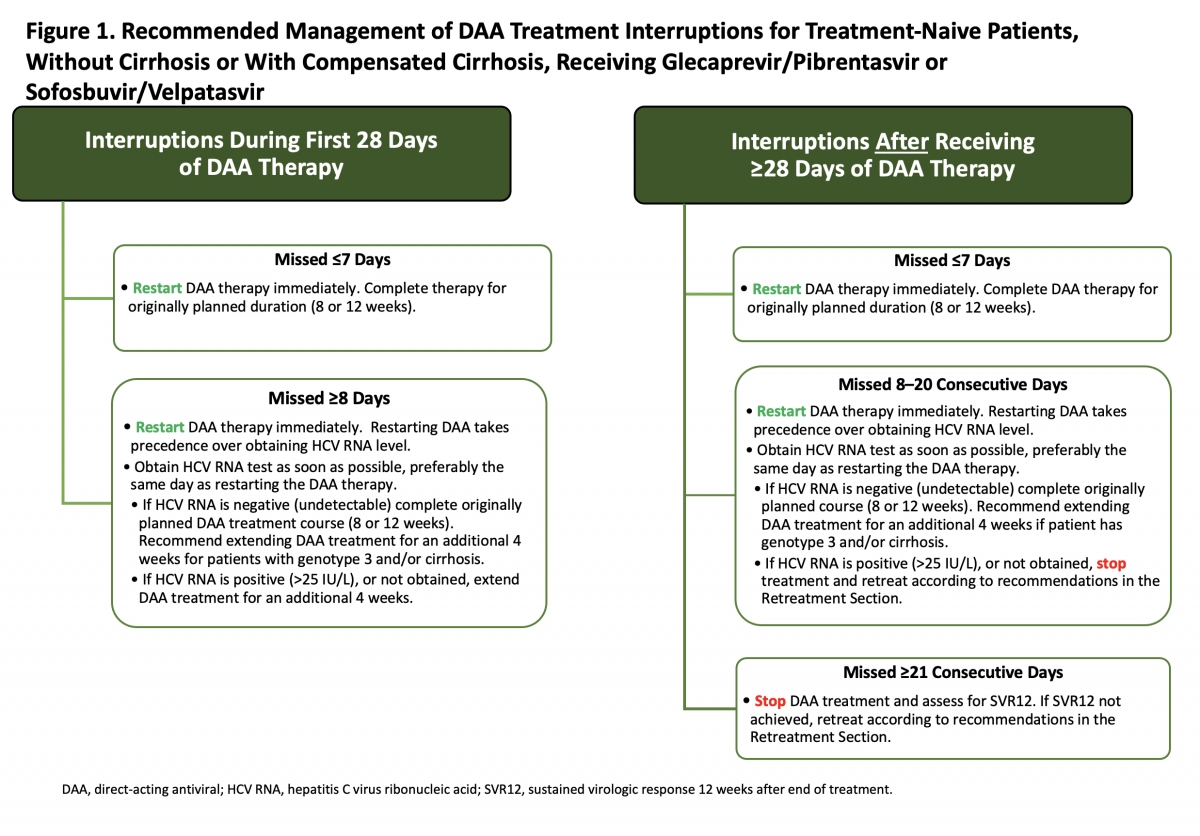
There are few data on which to base recommendations regarding how to manage patients who have discontinued DAAs for several days to weeks. The recommendations shown in Figure 1 are applicable to treatment-naive patients with acute or chronic HCV, without cirrhosis or without compensated cirrhosis, receiving either glecaprevir/pibrentasvir or sofosbuvir/velpatasvir. These recommendations are based on the opinion of the AASLD-IDSA HCV Treatment Guidance Panel.
Patients with prior DAA treatment, or receiving other DAA treatment regimens, or other populations (eg, patients who are posttransplant or have decompensated cirrhosis) should be managed in consultation with an expert. All patients with incomplete adherence should be asked about factors contributing to adherence or nonadherence, and counseled regarding the importance of adherence. In general, the panel considers a treatment interruption of <7 days unlikely to impact SVR12, based on adherence and outcome data from the SIMPLIFY study (Cunningham, 2018).
Click the graphic to enlarge Figure 1.