Test, Evaluate, Monitor
Testing, Evaluation, and Monitoring of Hepatitis C
The following pages address testing, evaluation, and monitoring of patients with HCV before, during and after antiviral therapy.
HCV Testing and Linkage to Care
One-Time Hepatitis C Testing
Recommendations for One-Time Hepatitis C Testing |
|
|---|---|
| RECOMMENDED |
RATING |
| One-time, routine, opt out HCV testing is recommended for all individuals aged 18 years or older. | I, B |
| One-time HCV testing should be performed for all persons less than 18 years old with activities, exposures, or conditions or circumstances associated with an increased risk of HCV infection (see below). | I, B |
| Prenatal HCV testing as part of routine prenatal care is recommended with each pregnancy. | I, B |
| Periodic repeat HCV testing should be offered to all persons with activities, exposures, or conditions or circumstances associated with an increased risk of HCV exposure (see below). | IIa, C |
| Annual HCV testing is recommended for all persons who inject drugs, for HIV-infected men who have unprotected sex with men, and men who have sex with men taking pre-exposure prophylaxis (PrEP). | IIa, C |
|
Risk Activities
Risk Exposures
Other Conditions and Circumstances
|
|
Based on the 2013–2016 National Health and Nutrition Examination Survey (NHANES) data among the general noninstitutionalized US population, an estimated 4.1 million people have had HCV exposure (HCV-antibody–positive), including 2.4 million with active HCV infection (HCV-RNA–positive) (Hofmeister, 2019). Total HCV burden in the US also includes those not accounted for in NHANES data—incarcerated, institutionalized, or unsheltered homeless persons—with estimates ranging from 380,000 to 800,000 additional HCV-antibody–positive persons (Hofmeister, 2019); (Edlin, 2015). Approximately 50% of all infected persons are unaware that they have HCV (Yehia, 2014); (Holmberg, 2013); (Denniston, 2012).
HCV screening is recommended because of the known benefits of care and treatment in reducing the risk of decompensated cirrhosis, hepatocellular carcinoma, and all-cause mortality, and the potential public health benefit of reducing transmission through early treatment, viral clearance, and reduced risk behaviors (Chou, 2020); (Owens, 2020); (Schillie, 2020); (Smith, 2012).
HCV is primarily transmitted through percutaneous exposure to infected blood. Other modes of transmission include mother-to-infant and contaminated devices shared for noninjection drug use. Sexual transmission also occurs but is generally inefficient except among HIV-infected men who have unprotected sex with men (Pakianathan, 2018).
Injection drug use (IDU) poses the greatest risk for HCV infection, accounting for at least 60% of acute HCV infections in the United States. Healthcare exposures are important sources of transmission, including: the receipt of blood products in the US prior to 1992 (after which routine screening of the blood supply was implemented); receipt of clotting factor concentrates in the US before 1987; receipt of blood or blood products in other countries (risk depends on country prevalence and screening practices); long-term hemodialysis; needlestick injuries among healthcare workers; and patient-to-patient transmission resulting from poor infection control practices. Other risk factors include having been born to an HCV-infected mother, incarceration, and percutaneous or parenteral exposures in an unregulated setting. Examples include tattoos received outside of licensed parlors and medical procedures performed internationally or domestically where strict infection control procedures may not have been followed (eg, surgery before implementation of universal precautions) (Hellard, 2004).
The importance of these risk factors might differ based on geographic location and population (Schillie, 2020); (Owens, 2020). An estimated 12% to 39% of incarcerated persons in North America are HCV-antibody–positive, supporting the recommendation to test this population for HCV infection (Larney, 2013); (Allen, 2003); (Weinbaum, 2003).
Because of shared transmission modes, persons with HIV infection are at risk for HCV. Annual HCV testing is recommended for sexually active HIV-infected adolescent and adult men who have sex with men. The presence of concomitant ulcerative sexually transmitted infections, proctitis related to sexually transmitted infections, or high-risk sexual or drug use practices may warrant more frequent testing. Sexual transmission is particularly a risk for HIV-infected men who have unprotected sex with men (Hosein, 2013); (van de Laar, 2010). Testing sexually active, non-HIV–infected persons for HCV and HBV infection before starting and while receiving pre-exposure prophylaxis (PrEP) for HIV prevention should also be considered (Hoornenborg, 2020); (Volk, 2015).
Data also support testing in all deceased and living solid organ donors and all recipients because of the risk of HCV infection posed to the recipient (Lai, 2013); (Jones, 2020). Although hepatitis C testing guidelines from the US Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF) do not specifically recommend testing immigrants from countries with a high HCV prevalence (eg, Egypt and Pakistan), such persons 18 years or older are included in the one-time, opt out HCV testing recommendation.
CDC established risk-based HCV testing guidelines in 1998 (CDC, 1998). These guidelines were expanded in 2012 with a recommendation to offer one-time HCV testing to all persons born from 1945 through 1965 without prior ascertainment of HCV risk factors. This recommendation was supported by evidence demonstrating that persons in this age group had a 6-fold higher prevalence of HCV infection and that a risk-based strategy alone failed to identify >50% of HCV infections, due in part to patient underreporting of their risk and provider limitations in ascertaining risk factor information (Denniston, 2012). The USPSTF also recommended one-time HCV testing in asymptomatic persons belonging to the 1945 through 1965 birth cohort as well as other individuals based on exposures, behaviors, and conditions or circumstances that increase HCV infection risk.
Since the birth cohort recommendation was adopted, however, there has been an increase in the number of acute and chronic HCV infections reported in individuals born after 1965 (Zibbell, 2018); (Ly, 2017); (Suryaprasad, 2014). The increase in HCV incidence and prevalence among a younger cohort results from the opioid epidemic and increased IDU. This shift in HCV epidemiology and the known failures of risk-based testing warranted an expansion of the recommendation. Accordingly, CDC updated screening recommendations in 2020 to include HCV screening at least once in a lifetime for all adults aged ≥18 years as well as HCV screening of all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection (ie, HCV-RNA positivity) is <0.1%. Both recommendations were based on extensive literature review and estimates of the cost-effectiveness of screening. In their recommendation, CDC noted that no states have an estimated HCV-RNA prevalence <0.1% (Schillie, 2020). USPSTF also recently issued recommendations for one-time, routine, opt-out testing of adults aged 18 through 79 years (Owens, 2020).
For the CDC 2020 testing guidelines, a systematic review included a harm assessment for HCV screening during pregnancy. This review was augmented by input from subject matter experts, studies not captured through the formal literature review, and the peer-review process. Despite several plausible harms (including insurability and employability issues, legal ramifications and potential loss of infant custody, unnecessary cesarean deliveries, and unnecessary avoidance of breastfeeding), CDC concluded that identified or potential harms did not outweigh the benefits of HCV screening (Schillie, 2020).
Generally, routine HCV testing is cost-effective because of increasing HCV incidence and prevalence among people who inject drugs (PWID) and the decreasing cost of DAA therapy. Many patients at greatest risk for HCV infection and transmission do not readily report their highly stigmatized risk activities. Studies conducted in US urban emergency departments, for example, reveal that 15% to 25% of patients with previously unidentified HCV infection were born after 1965 and/or have no reported history of IDU and are, therefore, missed by even perfect implementation of risk-based testing guidance (Schechter-Perkins, 2018); (Hsieh, 2016); (Lyons, 2016). Reinfection among those actively using drugs is common, but because HCV testing is a low-cost intervention and therapy is both highly effective and cost-effective, routine testing provides good economic value (ie, cost-effectiveness) even when many people need to be tested and treated more than once during their lifetime.
Several cost-effectiveness studies published since release of the birth cohort recommendations have demonstrated that routine, one-time HCV testing among all adults in the US would likely identify a substantial number of HCV cases that would otherwise be missed, and that doing so would be cost-effective. One research group employed simulation modeling to compare several versions of routine guidance, including routine testing for adults aged ≥40 years, ≥30 years, and ≥18 years. The investigators found that routine HCV testing for all adults ≥18 years was cost-effective compared to risk-based screening guidance, and potentially cost-saving compared to testing only those aged ≥30 years or ≥40 years (Barocas, 2018). The study further demonstrated that routine testing remained cost-effective unless HCV infection had no impact on healthcare utilization and no impact on quality of life. Another research team similarly found that routine HCV testing for all adults aged ≥18 years is likely cost-effective compared to risk-based screening guidance, provided the HCV prevalence among those born after 1965 is >0.07% (Eckman, 2019). Notably, these studies reached similar conclusions despite being conducted independently and employing different simulation modeling approaches. Further, a variety of studies have tested the cost-effectiveness of routine HCV testing in specific venues, including correctional settings (He, 2016), substance use treatment centers (Schackman, 2018); (Schackman, 2015), and federally qualified health centers (Assoumou, 2018). All of these studies demonstrated that routine HCV testing and treatment was cost-effective, even when linkage to HCV treatment after testing was poor and the rate of HCV reinfection among injection drug users was high.
Analyses focusing on pregnant women have demonstrated similar findings. One analysis calculated an incremental cost-effectiveness ratio (ICER) of $2,826 per quality-adjusted life-year (QALY) gained for universal screening of pregnant women compared with risk-based screening at an HCV-RNA positivity prevalence of 0.73% (Chaillon, 2019). Although real-world data informing screening during each pregnancy are lacking, a modeled analysis suggested that hepatitis C screening during each pregnancy would be cost-effective. Using a hepatitis C prevalence of 0.38% among pregnant women, the analysis found that universal hepatitis C screening during the first trimester of each pregnancy compared with the practice of risk-based screening had an ICER of $41,000 per QALY gained (Tasillo, 2019). Universal screening reduced HCV-attributable mortality by 16% and increased the proportion of infants identified as HCV-exposed from 44% to 92%. ICER remained ≤ $100,000 per QALY gained if hepatitis C prevalence was higher than 0.16% (Schillie, 2020).
Evidence regarding the frequency of HCV testing in persons at risk for ongoing exposures to the virus is lacking. Clinicians should, therefore, determine the periodicity of testing based on the risk of infection or reinfection. Because of the high incidence of HCV infection among PWID and HIV-infected men who have unprotected sex with men, HCV testing at least annually using an assay that detects HCV RNA (ie, a quantitative HCV-RNA test) if they have been previously exposed, is recommended among such individuals (Newsum, 2017); (Aberg, 2014); (Witt, 2013); (Bravo, 2012); (Linas, 2012); (Wandeler, 2012); (Williams, 2011).
Implementation of clinical decision support tools or prompts for HCV testing in electronic health records could facilitate reminding clinicians of HCV testing when indicated (Hsu, 2013); (Litwin, 2012).
Initial HCV Testing and Follow-Up
All persons for whom HCV screening is recommended should initially be tested for HCV antibody (CDC, 2013); (Alter, 2003) using an assay approved by the US Food and Drug Administration (FDA). A list of current FDA-approved HCV screening assays can be found on the agency website. FDA-approved tests include laboratory-based assays and a point-of-care assay (ie, OraQuick™ HCV rapid antibody test [OraSure Technologies]) (Lee, 2011). The latter is an indirect immunoassay with a sensitivity and specificity similar to those of laboratory-based HCV-antibody assays. Point-of-care assays are valuable in the community setting and allow for sample collection with a finger stick rather than standard phlebotomy. If point-of-care assays are used, reporting of results to the medical record and health authorities should follow protocols used for laboratory-based HCV-antibody tests. When possible, positive point-of-care antibody tests should be followed-up with immediate HCV-RNA confirmatory testing rather than referring the patient to another provider or setting to have the test performed. A study evaluating the performance parameters of the OraQuick™ HCV rapid antibody point-of-care test showed that people with viremia have higher antibody levels (compared with nonviremic persons), leading to a more rapid positive test result. All 227 viremic individuals in the study (from both clinical and real-world testing cohorts) tested positive within 5 minutes (Smookler, 2020). Based on a sensitivity of 100% (95% CI, 98.4-100%) in this study, if the OraQuick™ HCV rapid antibody test is not showing a positive result by 5 minutes, it is highly unlikely the person has active infection. Additional validation would be valuable, however, utilizing this so-called 5-minute rule may be considered, particularly in populations unable to wait the recommended 20–40 minutes before reading the test, or in high-throughput testing scenarios.
A positive HCV-antibody test indicates current (active) HCV infection (acute or chronic); past infection that has resolved; or a rare false positive (Pawlotsky, 2002). A test to detect HCV viremia is therefore necessary to confirm active HCV infection and guide clinical management, including initiation of HCV treatment. Many reference laboratories offer HCV-antibody testing that automatically reflexes to HCV-RNA PCR testing if the antibody test is positive. This should be considered the optimal testing approach in a clinical setting because it requires only a single blood draw without the need to bring people back to care for confirmatory testing, a major barrier in the continuum of care (Mera, 2016). HCV RNA point-of-care tests are also under evaluation (eg, Xpert® HCV viral load and Genedrive® HCV ID), which would allow for a rapid confirmation of viremia and immediate/same day treatment initiation. Point-of-care HCV-RNA tests are not yet FDA approved, as of this writing. Collection of dried blood spot (DBS) samples also allows for assessment of HCV antibodies and reflex HCV-RNA testing by testing spots sequentially. DBS samples can be collected using a finger stick rather than phlebotomy and can be transported without an intact cold chain, making it useful in rural areas and in people for whom phlebotomy may be a testing barrier (Lange, 2017).
HCV-RNA testing should also be performed in persons with a negative HCV-antibody test who are either immunocompromised (eg, persons receiving chronic hemodialysis) (KDIGO, 2008) or might have been exposed to HCV within the last 6 months because these persons may be HCV-antibody negative. An HCV-RNA test is also needed to detect reinfection in HCV-antibody–positive persons after previous spontaneous or treatment-related viral clearance.
Detection of HCV core antigen in the blood also indicates active HCV infection. Because the sensitivity of HCV core antigen testing is less than that of HCV-RNA testing, if an HCV core antigen test is used to assess viremia, antibody-positive samples that test negative for HCV core antigen should have a confirmatory HCV-RNA test to exclude a false negative core antigen result (van Tilborg, 2018).
An FDA-approved quantitative or qualitative HCV-RNA test with a detection level of ≤25 IU/mL should be used to detect HCV RNA. Figure 1 shows the CDC-recommended HCV testing algorithm.
Figure 1. CDC-Recommended Testing Sequence for Identifying Current HCV Infection
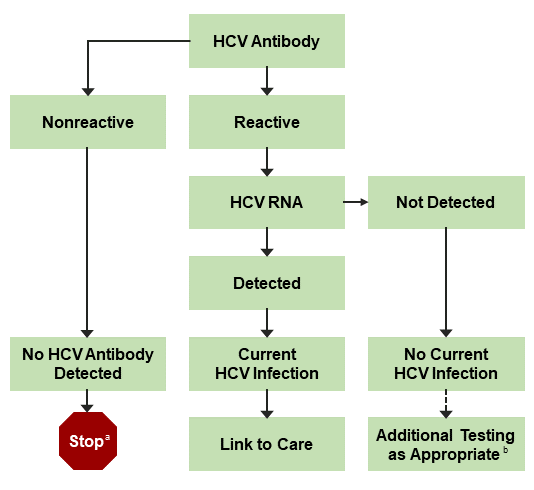
a For persons who might have been exposed to HCV within the past 6 months, testing for HCV RNA or follow-up testing for HCV antibody should be performed. For persons who are immunocompromised, testing for HCV RNA should be performed.
b To differentiate past, resolved HCV infection from biologic false positivity for HCV antibody, testing with another HCV-antibody assay can be considered. Repeat HCV-RNA testing if the person tested is suspected to have had HCV exposure within the past 6 months or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen.
Adapted from Centers for Disease Control and Prevention (CDC, 2013).
Persons who have a positive HCV-antibody test and negative results for HCV RNA by PCR should be informed that they do not have laboratory evidence of current HCV infection, although it is possible that they may have had a previous exposure. Additional HCV testing is typically unnecessary. The HCV-RNA test can be repeated when there is a high index of suspicion for recent infection or in patients with ongoing HCV infection risk. They should also be informed that despite the presence of antibodies, they are not protected from infection/reinfection.
Clinicians (or patients) may seek additional testing to determine whether a positive HCV-antibody test represents a remote, resolved HCV infection or a false positive. For patients with no apparent risk for HCV infection, the likelihood of a false positive HCV-antibody test is related to the HCV prevalence in the tested population. False positive HCV-antibody tests most commonly occur in populations with a low prevalence of HCV infection (Alter, 2003). If further testing is desired to distinguish between a true positive vs biologic false positivity for HCV antibody, repeat testing may be performed using a different FDA-approved, HCV-antibody assay. A biologic false result should not occur with 2 different assays because they target different regions of the virus, making it highly unlikely that both would falsely detect a cross-reactive antigen (CDC, 2013); (Vermeersch, 2008).
Prior to initiation of antiviral therapy, quantitative HCV-RNA testing should be used to determine the baseline level of viremia (ie, viral load), which may affect treatment duration with certain regimens. The degree of viral load decline after initiation of treatment is less predictive of sustained virologic response (SVR) in the era of direct-acting antiviral (DAA) therapy compared with previous interferon-based treatment (see Pretreatment and On-Treatment Monitoring).
With the advent of pangenotypic HCV treatment regimens, HCV genotyping is no longer required prior to treatment initiation for all individuals. In those with evidence of cirrhosis and/or past unsuccessful HCV treatment, treatment regimens may differ by genotype and thus pretreatment genotyping is recommended (see Treatment-Naive and Treatment-Experienced sections). For noncirrhotic treatment-naive patients, although genotyping may impact the preferred treatment approach, it is not required if a pangenotypic regimen is used (see Simplified Treatment Algorithm).
Counseling Persons With Active HCV Infection
Recommendations for Counseling Persons With Active HCV Infection |
|
|---|---|
| RECOMMENDED |
RATING |
| Persons with current HCV infection should receive education and interventions aimed at reducing liver disease progression and preventing HCV transmission. | IIa, B |
| Abstinence from alcohol and, when appropriate, interventions to facilitate cessation of alcohol consumption should be advised for all persons with HCV infection. | IIa, B |
| Evaluation for other conditions that may accelerate liver fibrosis, including hepatitis B and HIV infections, is recommended for all persons with active HCV infection. | IIb, B |
| Evaluation for advanced hepatic fibrosis using noninvasive tests (serum panels, elastography) or liver biopsy, if required, is recommended for all persons with HCV infection to facilitate an appropriate decision regarding HCV treatment strategy, and to determine the need for initiating additional measures for cirrhosis management (eg, hepatocellular carcinoma screening) (see Monitoring section). | I, A |
| Vaccination against hepatitis A and hepatitis B is recommended for all susceptible persons with HCV infection. | IIa, C |
| Vaccination against pneumococcal infection is recommended for all patients with cirrhosis. | IIa, C |
| All persons with HCV infection should be provided education about how to prevent HCV transmission to others. | I, C |
In addition to receiving antiviral therapy, HCV-infected persons should be educated about how to prevent further liver damage. Most important is prevention of the potentially deleterious effects of alcohol. Numerous studies have found a strong association between excess alcohol use and the development or progression of liver fibrosis, and the development of hepatocellular carcinoma (Safdar, 2004); (Harris, 2001); (Bellentani, 1999); (Corrao, 1998); (Wiley, 1998); (Poynard, 1997); (Noda, 1996). Daily consumption of >50 g of alcohol has a high likelihood of worsening fibrosis. Some studies indicate that daily consumption of lesser amounts of alcohol also exerts a deleterious effect on the liver; these data, however, are controversial (Hagström, 2017); (Younossi, 2013b); (Westin, 2002). Persons who abuse alcohol and have alcohol dependence require treatment and consideration for referral to an addiction specialist.
Hepatitis B virus (HBV) and HIV coinfection have been associated with a poorer HCV prognosis in cohort studies (Puoti, 2017b); (Kruse, 2014); (Thein, 2008a); (Zarski, 1998). Because of overlapping risk factors for these infections and benefits associated with their identification and treatment, HCV-infected persons should be tested for HIV antibody and hepatitis B surface antigen (HBsAg), using standard screening assays (Moyer, 2013); (CDC, 2008). See USPSTF HIV screening recommendations and CDC hepatitis B screening recommendations for additional information. Persons who test positive for HBsAg require monitoring during HCV treatment because of HBV reactivation risk (Lee, 2018). Anti-HBV therapy may also be considered (see reactivation of HBV in the Monitoring section). Persons who test negative for HBsAg but positive for hepatitis B core antibodies (anti-HBc)—with or without hepatitis B surface antibodies (anti-HBs)—have resolved HBV infection in most cases; the risk of clinically significant HBV reactivation with HCV therapy is very low in this scenario and no further workup is required (Mücke, 2018). Patients should be counseled about how to reduce their risk of acquiring these infections; HBV vaccination is recommended when appropriate.
Assessment of Liver Disease Severity
The severity of liver disease associated with chronic HCV infection is a key factor in determining the initial and follow-up evaluation of patients. Noninvasive tests using serum biomarkers, elastography, or liver imaging allow for accurate diagnosis of cirrhosis in most individuals (see pretreatment workup in When and in Whom to Initiate HCV Therapy). Liver biopsy is rarely required but may be considered if other causes of liver disease are suspected.
Noninvasive methods frequently used to estimate liver disease severity include:
- Liver-directed physical exam (normal in most patients)
- Routine blood tests (eg, ALT, AST, albumin, bilirubin, international normalized ratio [INR], and CBC with platelet count)
- Serum fibrosis marker panels
- Transient elastography
- Liver imaging (eg, ultrasound or CT scan)
Simple calculations derived from routine blood tests—such as the serum AST-to-platelet ratio index (APRI) (Wai, 2003) and FIB-4 score (Sterling, 2006)—as well as assessment of liver surface nodularity and spleen size by liver ultrasound or other cross-sectional imaging modalities can help determine if patients with HCV have cirrhosis and associated portal hypertension. The presence of portal hypertension is associated with a greater likelihood of developing future hepatic complications in untreated patients (Chou, 2013); (Rockey, 2006). Elastography provides instant information regarding liver stiffness and can reliably distinguish patients with a high versus low likelihood of cirrhosis (Bonder, 2014); (Castera, 2012). A more detailed discussion regarding fibrosis assessment is found in the When and In Whom to Initiate Therapy section.
Persons with known or suspected bridging fibrosis and cirrhosis are at increased risk for developing complications of advanced liver disease and require frequent follow-up. They should also avoid hepatotoxic drugs, such as excessive acetaminophen (>2 g/d) and certain herbal supplements. Nephrotoxic drugs, such as nonsteroidal anti-inflammatory drugs, should also be avoided. Ongoing imaging surveillance for liver cancer and gastroesophageal varices is recommended for these patients (Fontana, 2010); (Sangiovanni, 2006). Persons with cirrhosis are more susceptible to invasive pneumococcal infection (Marrie, 2011) and should receive pneumococcal vaccination (CDC, 2012).
Exposure to infected blood is the primary mode of HCV transmission. HCV-infected persons must be informed of the precautions needed to avoid exposing others to infected blood. This is particularly important for PWID given that HCV transmission in this population primarily results from sharing needles and other contaminated drug injection equipment. Epidemics of acute HCV due to sexual transmission in HIV-infected men who have sex with men have also been described (Urbanus, 2009); (van de Laar, 2009); (Fierer, 2008). Table 1 outlines measures to avoid HCV transmission. HCV is not spread by sneezing, hugging, holding hands, coughing, or sharing eating utensils or drinking glasses, nor is it transmitted through food or water.
Table 1. Measures to Prevent HCV Transmission
| HCV-infected persons should be counseled to avoid sharing toothbrushes and dental or shaving equipment, and be cautioned to cover any bleeding wound to prevent the possibility of others coming into contact with their blood. |
Persons should be counseled about harm reduction related to illicit drug use, including offering medication for opioid use disorder, if appropriate, or referral to a substance use treatment program. Those who continue to inject drugs should be referred to local syringe services programs and counseled to (Platt, 2017):
|
| Persons with HCV infection should be advised not to donate blood and to discuss HCV serostatus prior to donation of body organs, other tissue, or semen. |
| Persons with HIV infection and those with multiple sexual partners or sexually transmitted infections should be encouraged to use barrier precautions to prevent sexual transmission. Other persons with HCV infection should be counseled that the risk of sexual transmission is low and may not warrant barrier protection. |
| Household surfaces and implements contaminated with visible blood from an HCV-infected person should be cleaned using a dilution of 1 part household bleach to 9 parts water. Gloves should be worn when cleaning up blood spills. |
Improved identification of active HCV infection and treatment advances will have limited impact on HCV-related morbidity and mortality without concomitant improvement in linkage to care. All patients with current HCV infection and a positive HCV-RNA test should be evaluated by a healthcare provider with expertise in assessment of liver disease severity and HCV treatment. Subspecialty care and consultation may be required for persons with HCV infection who have advanced fibrosis or cirrhosis (Metavir stage ≥F3), including possible referral for consideration of liver transplantation in those with evidence of hepatic decompensation.
Data do not support exclusion of HCV-infected persons from consideration for hepatitis C therapy based on alcohol intake or use of illicit drugs (see Identification and Management of HCV in People Who Inject Drugs). Some possible strategies to address HCV treatment barriers are listed in Table 2.
Table 2. Common Barriers to and Misconceptions Regarding HCV Treatment and Potential Strategies
Barrier |
Strategy |
|---|---|
|
Comorbid conditions (eg, substance use, psychiatric disorders, uncontrolled chronic medical conditions) |
|
|
Competing priorities and loss to follow-up |
|
|
Treatment adherence and adverse effects |
|
|
Lack of access to treatment (eg, out-of-pocket costs, high copays, lack of insurance, geographic distance, and/or lack of specialist availability) |
|
|
Lack of practitioner expertise |
|
Co-localization of HCV screening, evaluation, and treatment with other medical or social services (ie, integrated care) is a strategy that addresses several treatment barriers. Co-localization has already been applied to settings with high HCV prevalence (eg, correctional facilities, needle exchange programs, substance abuse treatment centers, and harm reduction programs), but this type of care is not uniformly available (Burton, 2019); (Harrison, 2019); (Morey, 2019); (Schulkind, 2019); (Bruggmann, 2013); (Islam, 2012); (Stein, 2012). A recent study demonstrated that integrated care—consisting of multidisciplinary care coordination and patient case management—increased the proportion of patients with HCV infection and psychiatric illness or substance use who begin antiviral therapy and achieve SVR without serious adverse events (Ho, 2015).
A strategy that addresses lack of access to specialists—a primary barrier to HCV care—is participation in models involving close collaboration between primary care practitioners and subspecialists (Beste, 2017b); (Rossaro, 2013); (Miller, 2012); (Arora, 2011). Such collaborations have used telemedicine and knowledge networks to overcome geographic distances to specialists (Rossaro, 2013); (Arora, 2011) or the availability of experienced providers in a methadone or correctional setting (Morey, 2019); (Talal, 2019). For example, project ECHO (Extension for Community Healthcare Outcomes) uses videoconferencing to enhance primary care practitioner capacity in rendering HCV care and treatment to New Mexico's large rural and underserved population (Arora, 2011). Through case-based learning and real-time feedback from a multidisciplinary team of specialists (gastroenterology, infectious disease, pharmacology, and psychiatry practitioners), project ECHO has expanded HCV treatment access in populations that might have otherwise remained untreated. The short duration of treatment and few serious adverse events associated with DAA therapy present an opportunity to expand the number of primary care providers engaged in HCV management and treatment. This expansion will support the goal of HCV elimination and overcome barriers associated with the need for subspecialty referrals. The ASCEND trial utilized a real-world cohort of patients at urban federally qualified health centers and found that HCV treatment administered by nonspecialist providers was as safe and effective as that provided by specialists (Kattakuzhy, 2017).
Additional strategies for enhancing linkage to and retention in care could be adapted from other fields, such as tuberculosis and HIV. For example, use of directly observed therapy has enhanced adherence to tuberculosis treatment, and use of case managers and patient navigators has reduced loss of follow-up in HIV care (Govindasamy, 2012). Recent HCV testing and care programs have identified the use of patient navigators or care coordinators as important interventions in overcoming challenges associated with linkage to and retention in care (Ford, 2018); (Coyle, 2015); (Trooskin, 2015). There are also data suggesting that financial incentives and peer navigation may be useful to support treatment adherence in patients with substance use disorders (Ward, 2019); (Wohl, 2017). Ongoing assessment of efficacy and comparative effectiveness of this and additional strategies is a crucial area of future research for patients with HCV infection. Replication and expansion of best practices and new models for linkage to HCV care will also be crucial to maximize the public health impact of newer treatment paradigms.
When and in Whom to Initiate HCV Therapy
Successful hepatitis C treatment results in sustained virologic response (SVR), which is tantamount to virologic cure and, as such, is expected to benefit nearly all chronically infected persons. When the US Food and Drug Administration (FDA) approved the first interferon-sparing treatment for HCV infection, many patients who had previously been “warehoused” sought treatment. The infrastructure (ie, experienced practitioners, budgeted healthcare dollars, etc) did not yet exist to treat all patients immediately. Thus, the panel offered guidance for prioritizing treatment first for those with the greatest need.
Since that time, there have been opportunities to treat many of the highest-risk patients and accumulate real-world experience regarding the tolerability and safety of interferon-free HCV regimens. More importantly, from a medical standpoint, data continue to accumulate that demonstrate the many benefits, both intrahepatic and extrahepatic, that accompany HCV eradication. Therefore, the panel continues to recommend treatment for all patients with chronic HCV infection, except those with a short life expectancy that cannot be remediated by HCV treatment, liver transplantation, or another directed therapy. Accordingly, prioritization tables have been removed from this section.
Despite the strong recommendation for treatment of nearly all HCV-infected patients, pretreatment assessment of a patient’s understanding of treatment goals and provision of education about adherence and follow-up are essential. A well-established therapeutic relationship between clinician and patient remains crucial for optimal outcomes with direct-acting antiviral (DAA) therapies. Additionally, in certain settings there remain factors that impact access to medications and the ability to deliver them to patients. The descriptions of unique populations discussed in this section may help physicians make more informed treatment decisions for these groups. For additional information, see unique patient populations: Patients With HIV/HCV Coinfection; Patients With Decompensated Cirrhosis; Patients Who Develop Recurrent HCV Infection Post Liver Transplantation; Treatment of HCV-Uninfected Transplant Recipients Receiving Organs From HCV-Viremic Donors; Patients With Renal Impairment; HCV During Pregnancy; HCV in Children; Acute HCV Infection; and HCV Post Kidney Transplant.
Clinical Benefit of Cure
The proximate goal of HCV therapy is SVR (virologic cure), defined as the continued absence of detectable HCV RNA for at least 12 weeks after completion of therapy. SVR is a marker for cure of HCV infection and has been shown to be durable in large prospective studies in more than 99% of patients followed-up for ≥5 years (Manns, 2013); (Swain, 2010). While follow-up studies after cure using DAAs are limited, durability of SVR appears to be just as high (Reddy, 2018); (Sarrazin, 2017). Patients in whom SVR is achieved have HCV antibodies but no longer have detectable HCV RNA in serum, liver tissue, or mononuclear cells, and achieve substantial improvement in liver histology (Coppola, 2013); (Garcia-Bengoechea, 1999) (Marcellin, 1997). Assessment of viral response, including documentation of SVR, requires use of an FDA-approved quantitative or qualitative nucleic acid test (NAT) with a detection level of ≤25 IU/mL.
Patients who are cured of their HCV infection experience numerous health benefits, including a decrease in liver inflammation as reflected by improved aminotransferase levels (ie, alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), and a reduction in the rate of liver fibrosis progression (Poynard, 2002b). Among 3,010 treatment-naive patients from 4 randomized trials who had pretreatment and post-treatment liver biopsies (separated by a mean of 20 months) and were treated with 10 different interferon-based regimens, 39% to 73% of participants who achieved SVR had improvement in liver fibrosis and necrosis (Poynard, 2002b). Additionally, cirrhosis resolved in 49% of the cases. Portal hypertension, splenomegaly, and other clinical manifestations of advanced liver disease also improved. Among HCV-infected persons, SVR is associated with a >70% reduction in the risk of liver cancer (hepatocellular carcinoma [HCC]), and a 90% reduction in the risk of liver-related mortality and liver transplantation (Morgan, 2013); (van der Meer, 2012); (Veldt, 2007).
Cure of HCV infection also reduces symptoms and mortality from severe extrahepatic manifestations, including cryoglobulinemic vasculitis, a condition affecting 10% to 15% of HCV-infected patients (Sise, 2016); (Fabrizi, 2013); (Landau, 2010). HCV-infected persons with non-Hodgkin lymphoma and other lymphoproliferative disorders achieve complete or partial remission in up to 75% of cases following successful therapy for HCV infection (Takahashi, 2012); (Gisbert, 2005); (Svoboda, 2005); (Hermine, 2002); (Mazzaro, 2002). These reductions in disease severity contribute to dramatic reductions in all-cause mortality (van der Meer, 2012); (Backus, 2011). Furthermore, patients who achieve SVR have a substantially improved quality of life, which spans their physical, emotional, and social health (Gerber, 2016); (Boscarino, 2015); (Younossi, 2014b); (Neary, 1999). Conversely, patients who do not achieve SVR after treatment have a continued worsening in health-related quality of life (Younossi, 2019).
Despite convincing data from observational studies demonstrating the benefit of SVR on all-cause and liver-related mortality, the lack of randomized, placebo-controlled trials of HCV DAA treatment focusing on clinical endpoints (eg, mortality, HCC, liver decompensation, etc) and reliance on surrogate endpoints (eg, HCV RNA) have led some to question the benefits of HCV treatment. In further support of the dramatic benefit of HCV cure, a French cohort study that prospectively followed almost 10,000 patients with chronic HCV infection (including 2,500 who remained untreated for HCV) for a median of 33 months demonstrated a 52% reduction in all-cause mortality and a 34% reduction in HCC (Carrat, 2019).
Because of the many benefits associated with successful HCV treatment, clinicians should treat HCV-infected patients with antiviral therapy with the goal of achieving SVR, preferably early in the course of chronic hepatitis C before the development of severe liver disease and other complications.
Benefits of Treatment at Early Fibrosis Stages (Metavir Stage Less Than F2)
Initiating therapy in patients with lower-stage fibrosis augments the benefits of SVR. In a long-term follow-up study, 820 patients with biopsy-confirmed Metavir stage F0 or F1 fibrosis were followed for up to 20 years (Jezequel, 2015). The 15-year survival rate was significantly better for those who experienced SVR than for those whose treatment failed or those who remained untreated (93%, 82%, and 88%, respectively; P =.003). The study results argue for consideration of earlier initiation of treatment. Several modeling studies also suggest a greater mortality benefit if treatment is initiated at fibrosis stages prior to F3 (Matsuda, 2016); (Zahnd, 2015); (Øvrehus, 2015).
Treatment delay may decrease the benefit of SVR. In a report from France, 820 patients with biopsy-confirmed Metavir stage F0 or F1 fibrosis were followed for as long as 20 years (Jezequel, 2015). The authors noted rapid progression of fibrosis in 15% of patients during follow-up, and in patients treated successfully, long-term survival was better. Specifically, at 15 years, survival rate was 92% for those with SVR versus 82% for treatment failures and 88% for those not treated. In a Danish regional registry study, investigators modeled treatment approaches with the aim of evaluating the benefit to the region in terms of reductions in morbidity and mortality and HCV prevalence (Øvrehus, 2015). Although they note that in their situation of low HCV prevalence (0.4%) with approximately 50% undiagnosed, a policy that restricts treatment to those with Metavir fibrosis stage F3 or higher would decrease mortality from HCC and cirrhosis, the number needed to treat to halve the prevalence of the disease is lower if all eligible patients receive treatment at diagnosis.
A modeling study based on the Swiss HIV cohort study also demonstrated that waiting to treat HCV infection until Metavir fibrosis stages F3 and F4 resulted in 2- and 5-times higher rates of liver-related mortality, respectively, compared with treating at Metavir stage F2 (Zahnd, 2015). A US Veterans Administration dataset analysis that used very limited endpoints of virologic response dating from the interferon-treatment era suggested that early initiation of therapy (at a fibrosis-4 [FIB-4] score of <3.25) increased the benefit attained with respect to likelihood of treatment success and mortality reduction, and ultimately decreased the number of patients needed to treat to preserve 1 life by almost 50% (Matsuda, 2016).
Considerations in Specific Populations
Despite the recommendation for treatment of nearly all patients with HCV infection, it remains important for clinicians to understand patient- and disease-related factors that place individuals at risk for HCV-related complications (liver and extrahepatic) as well as for HCV transmission. Although these groups are no longer singled out for high prioritization for treatment, it is nonetheless important that clinicians recognize the unique dimensions of HCV disease and its natural history in these populations. The discussions offered below may assist clinicians in making compelling cases for insurance coverage of treatment when necessary.
Persons With Advanced Liver Disease
For persons with advanced liver disease (Metavir stage F3 or F4), the risk of developing complications of liver disease, such as hepatic decompensation (Child-Turcotte-Pugh [CTP] class B or C [Methods Table 3] ![]() ) or HCC, is substantial and may occur in a relatively short timeframe. A large prospective study of patients with cirrhosis resulting from HCV infection examined the risk of decompensation—including HCC, ascites, jaundice, bleeding, and encephalopathy—and found that the overall annual incidence rate was 3.9% (Sangiovanni, 2006). The National Institutes of Health (NIH)-sponsored HALT–C study included a group of 220 patients with HCV-related cirrhosis who were observed for approximately 8 years. A primary outcome of death, hepatic decompensation, HCC, or an increase in CTP score ≥2 occurred at a rate of 7.5% per year (Di Bisceglie, 2008); (Everson, 2006). Patients with a CTP score of ≥7 experienced a death rate of 10% per year.
) or HCC, is substantial and may occur in a relatively short timeframe. A large prospective study of patients with cirrhosis resulting from HCV infection examined the risk of decompensation—including HCC, ascites, jaundice, bleeding, and encephalopathy—and found that the overall annual incidence rate was 3.9% (Sangiovanni, 2006). The National Institutes of Health (NIH)-sponsored HALT–C study included a group of 220 patients with HCV-related cirrhosis who were observed for approximately 8 years. A primary outcome of death, hepatic decompensation, HCC, or an increase in CTP score ≥2 occurred at a rate of 7.5% per year (Di Bisceglie, 2008); (Everson, 2006). Patients with a CTP score of ≥7 experienced a death rate of 10% per year.
Numerous studies have demonstrated that hepatitis C therapy and the achievement of SVR in this population results in dramatic decreases in hepatic decompensation events, HCC, and liver-related mortality (Mira, 2013); (Morgan, 2013); (van der Meer, 2012); (Backus, 2011); (Dienstag, 2011); (Berenguer, 2009). In the HALT-C study, patients with advanced fibrosis secondary to HCV infection who achieved SVR, compared with patients with similarly advanced liver fibrosis who did not achieve SVR, had a decreased need for liver transplantation (HR, 0.17; 95% CI, 0.06-0.46), decreased development of liver-related morbidity and mortality (HR, 0.15; 95% CI, 0.06-0.38), and decreased HCC (HR, 0.19; 95% CI, 0.04-0.80) (Dienstag, 2011). Importantly, persons with advanced liver disease also require long-term follow-up and HCC surveillance regardless of treatment outcome (see Monitoring Patients Who Are Starting Hepatitis C Treatment, Are on Treatment, or Have Completed Therapy).
Given the clinical complexity and need for close monitoring, patients with advanced liver disease that has already decompensated (CTP class B or C [Methods Table 3] ![]() ) should be treated by physicians with experience treating HCV in conjunction with a liver transplantation center, if possible (see Patients with Decompensated Cirrhosis).
) should be treated by physicians with experience treating HCV in conjunction with a liver transplantation center, if possible (see Patients with Decompensated Cirrhosis).
Persons Who Have Undergone Liver Transplantation
In HCV-infected individuals, HCV infection of the liver allograft occurs universally in those with viremia at the time of transplantation. Histologic features of hepatitis develop in about 75% of recipients within the first 6 months following liver transplantation (Neumann, 2004). By the fifth postoperative year, up to 30% of untreated patients have progressed to cirrhosis (Neumann, 2004); (Charlton, 1998). A small proportion of patients (4% to 7%) develop an accelerated course of liver injury (cholestatic hepatitis C, associated with very high levels of viremia) with subsequent rapid allograft failure. Recurrence of HCV infection post transplantation is associated with decreased graft survival for recipients with HCV infection compared to recipients who undergo liver transplantation for other indications (Forman, 2002).
Effective HCV therapy prior to transplantation resulting in SVR (virologic cure) prevents HCV recurrence post transplantation (Everson, 2003). In addition, complete HCV viral suppression prior to transplantation prevents recurrent HCV infection of the graft in the majority of cases (Everson, 2005); (Forns, 2004). Preliminary data from a study of patients with complications of cirrhosis secondary to HCV infection who were wait-listed for liver transplantation (included patients with MELD scores up to 14 and CTP scores up to 8) found that treatment with sofosbuvir and weight-based ribavirin for up to 48 weeks was well tolerated and associated with an overall SVR of 70% post transplant (Curry, 2015). Post-transplant SVR was nearly universal among patients who had undetectable HCV RNA for 28 days or longer prior to transplantation.
Treatment of established HCV infection post transplantation also yields substantial improvements in patient and graft survival (Berenguer, 2008); (Picciotto, 2007). The availability of effective, interferon-free antiviral therapy has addressed the major hurdles to treating HCV recurrence post transplantation—poor tolerability and efficacy. A multicenter, open-label study evaluated the efficacy of sofosbuvir plus ribavirin to induce virologic suppression in 40 patients after liver transplantation with compensated recurrence of HCV infection. Daily sofosbuvir plus ribavirin for 24 weeks achieved SVR12 in 70% of these patients (Charlton, 2015). No deaths, graft losses, or episodes of rejection occurred. Six patients had serious adverse events, all of which were considered unrelated to the study treatment. There were no drug interactions reported between sofosbuvir and any of the concomitant immunosuppressive agents. In contrast, treatment with sofosbuvir plus ribavirin, with or without peginterferon, in 64 patients with severe, decompensated cirrhosis resulting from recurrence of HCV infection following liver transplantation was associated with an overall SVR12 of 59% and a mortality rate of 13% (Forns, 2015). On an intent-to-treat basis, treatment was associated with clinical improvement in 57% and stable disease in 22% of patients. Given the clinical complexity (including drug-drug interactions and the need for close monitoring), patients with a liver transplant should be treated by physicians with experience in treating this population (see Patients Who Develop Recurrent HCV Infection Post Liver Transplantation).
Persons at Increased Risk for Rapidly Progressive Fibrosis and Cirrhosis
Fibrosis progression is variable across different patient populations as well as within the same individual over time. Many of the components that determine fibrosis progression and development of cirrhosis in an individual are unknown. However, certain factors, such as coinfection with HIV or the hepatitis B virus (HBV) and prevalent coexistent liver diseases (eg, nonalcoholic steatohepatitis [NASH]), are well recognized contributors to accelerated fibrosis progression (see Table below).
HIV/HCV Coinfection
HIV coinfection accelerates fibrosis progression among HCV-infected persons (Konerman, 2014); (Macias, 2009); (Benhamou, 1999), although control of HIV replication and restoration of the CD4 cell count may mitigate this to some extent but the effect is not completely reversed (Lo Re, 2014); (Bräu, 2006); (Benhamou, 2001). Thus, antiretroviral therapy is not a substitute for HCV treatment. In the largest paired-biopsy study, 282 HIV/HCV-coinfected patients with 435 paired biopsies were prospectively evaluated (Konerman, 2014). Thirty-four percent of patients showed fibrosis progression of at least 1 Metavir stage at a median of 2.5 years. Importantly, 45% of patients with no fibrosis on initial biopsy had progression. Finally, a more rapid progression to death following decompensation combined with lack of widespread access to liver transplantation and poor outcomes following transplantation highlight the need for HCV treatment in this population regardless of current fibrosis stage (see Patients with HIV/HCV Coinfection) (Terrault, 2012); (Merchante, 2006); (Pineda, 2005).
HBV/HCV Coinfection
The prevalence of HBV/HCV coinfection is estimated at 1.4% in the United States and 5% to 10% globally (Tyson, 2013); (Chu, 2008). Persons with HBV/HCV coinfection and detectable viremia of both viruses are at increased risk for disease progression, decompensated liver disease, and the development of HCC. HBV/HCV-coinfected individuals are susceptible to a process called viral interference wherein one virus may interfere with the replication of the other virus. Thus, when treating one or both viruses with antiviral drugs, periodic retesting of HBV DNA and HCV RNA levels during and after therapy is prudent, particularly if only one of the viruses is being treated at a time. Treatment of HCV infection in such cases utilizes the same genotype-specific regimens as are recommended for HCV monoinfection (see Initial Treatment of HCV Infection). HBV infection in such cases should be treated as recommended for HBV monoinfection (Lok, 2009).
Other Coexistent Liver Diseases
Persons with other chronic liver diseases who have coincident chronic HCV infection should be considered for HCV therapy given the potential for rapid liver disease progression. An interferon-free regimen is preferred for immune-mediated liver diseases, such as autoimmune hepatitis, because of the potential for interferon-related exacerbation.
Persons With Extrahepatic Manifestations of Chronic HCV Infection
Cryoglobulinemia
Chronic hepatitis C is associated with a syndrome of cryoglobulinemia, an immune complex and lymphoproliferative disorder that leads to arthralgia, fatigue, palpable purpura, renal disease (eg, membranoproliferative glomerulonephritis), neurologic disease (eg, peripheral neuropathy, central nervous system vasculitis), and reduced complement levels (Agnello, 1992). Glomerular disease results from deposition of HCV-related immune complexes in the glomeruli (Johnson, 1993). Because patients with chronic hepatitis C frequently have laboratory evidence of cryoglobulins (>50% in some series), antiviral treatment is imperative for those with the syndrome of cryoglobulinemia and symptoms or objective evidence of end-organ manifestations. Limited data with DAA therapy in the setting of vasculitis end-organ disease related to cyroglobulinemia have demonstrated responses in 20% to 90% of patients (Comarmond, 2017); (Emery, 2017). Despite this, patients with severe end-organ disease may still require treatment with plasmapheresis or rituximab (Emery, 2017).
Diabetes
The relationship between chronic hepatitis C and diabetes (most notably type 2 diabetes and insulin resistance) is complex and incompletely understood. The prevalence and incidence of diabetes is increased in the context of hepatitis C (White, 2008). In the United States, type 2 diabetes occurs more frequently in HCV-infected patients, with a >3-fold greater risk in persons older than 40 years (Mehta, 2000). The positive correlation between plasma HCV RNA load and established markers of insulin resistance confirms this relationship (Yoneda, 2007). Insulin resistance and type 2 diabetes are independent predictors of accelerated liver fibrosis progression (Petta, 2008). Patients with type 2 diabetes and insulin resistance are also at increased risk for HCC (Hung, 2010).
Successful antiviral treatment has been associated with improved markers of insulin resistance and a greatly reduced incidence of new-onset type 2 diabetes and insulin resistance in HCV-infected patients (Arase, 2009). Most recently, HCV antiviral therapy has been shown to improve clinical outcomes related to diabetes. In a large prospective cohort from Taiwan, the incidence rates of end-stage renal disease, ischemic stroke, and acute coronary syndrome were greatly reduced in HCV-infected patients with diabetes who received antiviral therapy compared to untreated, matched controls (Hsu, 2014). Therefore, antiviral therapy may prevent progression to diabetes in HCV-infected patients with prediabetes, and may reduce renal and cardiovascular complications in HCV-infected patients with established diabetes.
Fatigue
Fatigue is the most frequently reported symptom in patients with chronic hepatitis C, and has a major effect on quality of life and activity level as evidenced by numerous measures of impaired quality of life (Foster, 1998). The presence and severity of fatigue appears to correlate poorly with disease activity, although it may be more common and severe in HCV-infected individuals with cirrhosis (Poynard, 2002a). Despite difficulties in separating fatigue symptoms associated with hepatitis C from those associated with other concurrent conditions (eg, anemia, depression), numerous studies have reported a reduction in fatigue after cure of HCV infection (Bonkovsky, 2007). In the Virahep-C study, 401 patients with HCV infection were evaluated for fatigue prior to and after treatment, using validated scales to assess the presence and severity of fatigue (Sarkar, 2012). At baseline, 52% of patients reported having fatigue, which was more frequent and severe in patients with cirrhosis than in those without cirrhosis. Achieving SVR was associated with a substantial decrease in the frequency and severity of fatigue.
A recent analysis of 413 patients from the NEUTRINO and FUSION trials who were treated with a sofosbuvir-containing regimen and achieved SVR12 demonstrated improvement in patient fatigue (present in 12%) from the pretreatment level (Younossi, 2014). After achieving SVR12, participants had marked improvements in fatigue over their pretreatment scores, measured by 3 separate validated questionnaires. Additional studies support and extend these findings beyond fatigue, with improvements in overall health-related quality of life and work productivity observed following successful HCV therapy (Gerber, 2016); (Younossi, 2016a); (Younossi, 2015b); (Younossi, 2015c); (Younossi, 2015d); (Younossi, 2015e).
Dermatologic Manifestations
The reported prevalence of HCV infection in patients with porphyria cutanea tarda approximates 50% and occurs disproportionately in those with cirrhosis (Gisbert, 2003). The treatment of choice for active porphyria cutanea tarda is iron reduction by phlebotomy and maintenance of a mildly iron-reduced state without anemia. Although improvement of porphyria cutanea tarda during HCV treatment with interferon has frequently been described (Takikawa, 1995), there are currently insufficient data to determine whether HCV DAA therapy and achievement of SVR results in porphyria cutanea tarda improvement.
Lichen planus is characterized by pruritic papules involving mucous membranes, hair, and nails. HCV antibodies are present in 10% to 40% of patients with lichen planus but a causal link with chronic HCV infection is not established. Resolution of lichen planus has been reported with interferon-based regimens, but there have also been reports of exacerbation with these treatments. Although it is unknown whether DAAs will have more success against lichen planus, treatment with interferon-free regimens would appear to be a more advisable approach to addressing this disorder (Gumber, 1995); (Sayiner, 2017).
Benefit of Treatment to Reduce Transmission
Persons who have successfully achieved SVR (virologic cure) no longer transmit the virus to others. As such, successful treatment of HCV infection benefits public health. Several health models have shown that even modest increases in successful treatment of HCV infection among persons who inject drugs can decrease prevalence and incidence (Harris, 2016); (Martin, 2013a); (Martin, 2013b); (Durier, 2012); (Hellard, 2012). Models developed to estimate the impact of HCV testing and treatment on the burden of hepatitis C at a country level reveal that large decreases in HCV prevalence and incidence are possible as more persons are successfully treated (Wedemeyer, 2014).
There are also benefits to eradicating HCV infection between couples and among families, thus eliminating the perception that an individual might be contagious. In addition, mother-to-child transmission of HCV does not occur if the woman is not viremic, providing an additional benefit of curing a woman before she becomes pregnant (Thomas, 1998). The safety and efficacy of treating women who are already pregnant, however, to prevent transmission to the fetus have not yet been established. Thus, treatment is not recommended for pregnant women.
The Society for Healthcare Epidemiology of America (SHEA) advises that healthcare workers who have substantial HCV viral replication (≥104 genome equivalents/mL) be restricted from performing procedures that are prone to exposure (Henderson, 2010) and that all healthcare workers with confirmed chronic HCV infection should be treated. For reasons already stated, the achievement of SVR in such individuals will not only eliminate the risk of HCV transmission to patients but also decrease circumstantial loss of experienced clinicians. Given concerns about underreporting of infection and transmission (Henderson, 2010), the availability of effective, all-oral regimens should lead to greater willingness on the part of exposure-prone clinicians to be tested and treated.
Successful treatment of HCV-infected persons at greatest risk for transmission represents a formidable tool to help stop HCV transmission in those who continue to engage in high-risk behaviors. To guide implementation of hepatitis C treatment as a prevention strategy, studies are needed to define the best candidates for treatment to stop transmission, the additional interventions needed to maximize the benefits of HCV treatment (eg, preventing reinfection), and the cost-effectiveness of the strategies when used in target populations.
Persons Who Inject Drugs
Injection drug use (IDU) is the most common risk factor for HCV infection in the United States and Europe, with an HCV seroprevalence rate of 10% to 70% (Amon, 2008); (Nelson, 2011). IDU also accounts for the majority of new HCV infections (approximately 70%) and is the key driving force in the perpetuation of the epidemic. Given these facts and the absence of an effective vaccine against HCV, testing and linkage to care combined with treatment of HCV infection with potent DAAs has the potential to dramatically decrease HCV incidence and prevalence (Martin, 2013b). However, treatment-based strategies to prevent HCV transmission have yet to be studied, including how to integrate hepatitis C treatment with other risk-reduction strategies (eg, opiate substitution therapy, and needle and syringe exchange programs) (Martin, 2013a).
In studies of interferon-based treatments in persons who inject drugs, adherence and efficacy rates are comparable to those of patients who do not use injected drugs. A meta-analysis of treatment with peginterferon, with or without ribavirin, in active or recent injection drug users showed SVR rates of 37% and 67% for genotype 1 or 4, and 2 or 3, respectively (Aspinall, 2013). With the introduction of shorter, better-tolerated, and more efficacious interferon-free therapies, these SVR rates are expected to improve. Importantly, the rate of reinfection in this population is lower (2.4/100 person-years of observation) than that of incident infection in the general population of injection drug users (6.1 to 27.2/100 person-years), although reinfection increases with active or ongoing IDU (6.44/100 person-years) and available data on follow-up duration are limited (Aspinall, 2013); (Grady, 2013).
Ideally, treatment of HCV-infected persons who inject drugs should be delivered in a multidisciplinary care setting with services to reduce the risk of reinfection and for management of the common social and psychiatric comorbidities in this population (Dore, 2016); (Mathei 2016); (Midgard 2016); (Murphy 2015). Regardless of the treatment setting, recent or active IDU should not be seen as an absolute contraindication to HCV therapy. There is strong evidence from various settings in which persons who inject drugs have demonstrated adherence to treatment and low rates of reinfection, countering arguments that have been commonly used to limit treatment access in this patient population (Hellard, 2014); (Aspinall, 2013); (Grebely, 2011). Indeed, combining HCV treatment with needle exchange and opioid agonist therapy programs in this population with a high prevalence of HCV has shown great value in decreasing the burden of HCV disease. Elegant modeling studies illustrate high return on the modest investment of addressing this often-ignored segment of the HCV-infected population (Martin, 2013b). These conclusions were drawn before the introduction of the latest DAA regimens. Conversely, there are no data to support the utility of pretreatment screening for illicit drug or alcohol use in identifying a population more likely to successfully complete HCV therapy. These requirements should be abandoned because they create barriers to treatment, add unnecessary cost and effort, and potentially exclude populations that are likely to obtain substantial benefit from therapy. Scaling up HCV treatment in persons who inject drugs is necessary to positively impact the HCV epidemic in the US and globally.
HIV-Infected Men Who Have Sex With Men
Since 2000, a dramatic increase in incident HCV infections among HIV-infected men who have sex with men (MSM) who did not report IDU as a risk factor has been demonstrated in several US cities (Samandari, 2017); (van de Laar, 2010). Recognition and treatment of HCV infection (including acute infection) in this population may represent an important step in preventing subsequent infections (Martin, 2016). As with persons who inject drugs, HIV/HCV-coinfected MSM who engage in ongoing high-risk sexual practices should be treated for their HCV infection in conjunction with continued education about risk-reduction strategies. In particular, safer-sex strategies should be emphasized given the high rate of reinfection after SVR, which may approach 30% over 2 years in HIV-infected MSM with acute HCV infection (Lambers, 2011).
Some of the best examples of HCV treatment as prevention of transmission have come from well characterized cohorts of HIV/HCV coinfected MSM. In the Dutch acute HCV in HIV study (DAHHS) cohort, a 51% decrease in HCV incidence among MSM living with HIV was realized in just 2 years after implementing a comprehensive HCV screening and immediate treatment program (Boerekamps, 2017). Similarly, in the Swiss HIV cohort study (SHCS), a 92.5% reduction in HCV prevalence and 51% decrease in incident HCV infections was realized shortly after implementing universal screening and treatment within an MSM cohort living with HIV (Braun, 2018).
Incarcerated Persons
Among incarcerated individuals, the rate of HCV seroprevalence ranges from 30% to 60% (Post, 2013) and the rate of acute infection is approximately 1% (Larney, 2013). Screening for HCV infection is relatively uncommon in state prison systems. Treatment uptake has historically been limited, in part because of the toxic effects and long treatment duration of older interferon-based therapies as well as cost concerns (Spaulding, 2006). In particular, truncation of HCV treatment owing to release from prison has been cited as a major limitation to widespread, effective HCV treatment in correctional facilities (Post, 2013); (Chew, 2009). Shorter HCV treatment duration with DAA regimens reduces stay-related barriers to HCV treatment in prisons. Likewise, the improved safety of DAA regimens diminishes concerns about toxic effects. Coordinated treatment efforts within prison systems would likely rapidly decrease HCV prevalence in this at-risk population (He, 2016), although research is needed in this area.
Persons on Hemodialysis
HCV prevalence is markedly elevated in persons on hemodialysis, ranging from 2.6% to 22.9% in a large multinational study (Fissell, 2004). US studies found a similarly elevated prevalence of 7.8% to 8.9% (Finelli, 2005); (CDC, 2001). Importantly, the seroprevalence of HCV was found to increase with time on dialysis, suggesting that nosocomial transmission, among other risk factors, plays a role in HCV acquisition in these patients (Fissell, 2004). Improved education and strict adherence to universal precautions can drastically reduce nosocomial HCV transmission risk for persons on hemodialysis (Jadoul, 1998), but clearance of HCV viremia through treatment-induced SVR eliminates the potential for transmission.
HCV-infected persons on hemodialysis have a decreased quality of life and increased mortality compared to those who are uninfected (Fabrizi, 2009); (Fabrizi, 2007); (Fabrizi, 2002). HCV infection in this population also has a deleterious impact on kidney transplantation outcomes with decreased patient and graft survival (Fabrizi, 2014). The increased risk for nosocomial transmission and the substantial clinical impact of HCV infection in those on hemodialysis are compelling arguments for HCV therapy as effective antiviral regimens that can be used in persons with advanced renal failure are now available (see Patients with Renal Impairment).
Patients Unlikely to Benefit From HCV Treatment
Patients with a limited life expectancy that cannot be remediated by HCV treatment, liver transplantation, or another directed therapy do not require antiviral treatment. Patients with a short life expectancy owing to liver disease should be managed in consultation with an expert. Chronic hepatitis C is associated with a wide range of comorbid conditions (Louie, 2012); (Butt, 2011). Little evidence exists to support initiation of HCV treatment in patients with a limited life expectancy (<12 months) owing to nonliver-related comorbid conditions. For these patients, the benefits of HCV treatment are unlikely to be realized and palliative care strategies should take precedence (Maddison, 2011); (Holmes, 2006).
Pretreatment Assessment
Recommendation for Pretreatment Assessment |
|
|---|---|
| RECOMMENDED |
RATING |
| Evaluation for advanced fibrosis using noninvasive markers and/or elastography, and rarely liver biopsy, is recommended for all persons with HCV infection to facilitate decision making regarding HCV treatment strategy and determine the need for initiating additional measures for the management of cirrhosis (eg, hepatocellular carcinoma screening) (see HCV Testing and Linkage to Care). | I, A |
An accurate assessment of fibrosis remains vital as the degree of hepatic fibrosis is one of the most robust prognostic factors used to predict HCV disease progression and clinical outcomes (Everhart, 2010). Individuals with severe fibrosis require surveillance monitoring for liver cancer, esophageal varices, and hepatic function (Bruix, 2011); (Garcia-Tsao, 2007). In some instances, the recommended duration of treatment is also longer.
Although liver biopsy is the diagnostic standard, sampling error and observer variability limit test performance, particularly when inadequate sampling occurs. Up to 1/3 of bilobar biopsies had a difference of at least 1 stage between the lobes (Bedossa, 2003). In addition, the test is invasive and minor complications are common, limiting patient and practitioner acceptance. Although rare, serious complications such as bleeding are well recognized.
Noninvasive tests to stage the degree of fibrosis in patients with chronic HCV infection include models incorporating indirect serum biomarkers (routine tests), direct serum biomarkers (components of the extracellular matrix produced by activated hepatic stellate cells), and vibration-controlled transient liver elastography. No single method is recognized to have high accuracy alone, and each test must be interpreted carefully. A publication from the Agency for Healthcare Research and Quality found evidence in support of a number of blood tests; however, at best, they are only moderately useful for identifying clinically significant fibrosis or cirrhosis (Selph, 2014).
Vibration-controlled transient liver elastography is a noninvasive way to measure liver stiffness and correlates well with measurement of substantial fibrosis or cirrhosis in patients with chronic HCV infection. The measurement range, however, overlaps between stages (Afdhal, 2015); (Castera, 2005); (Ziol, 2005).
The most efficient approach to fibrosis assessment is to combine direct biomarkers and vibration-controlled transient liver elastography (European Association for the Study of the Liver and Asociacion Latinoamericana para el Estudio del Higado, 2015); (Boursier, 2012). A biopsy should be considered for any patient who has discordant results between the 2 modalities that would affect clinical decision making (eg, one shows cirrhosis and the other does not). The need for liver biopsy with this approach is markedly reduced.
Alternatively, if direct biomarkers or vibration-controlled transient liver elastography are not available, the AST-to-platelet ratio index (APRI) or FIB-4 index score can prove helpful—although neither is sensitive enough to rule out substantial fibrosis (Chou, 2013); (Castera, 2010); (Sebastiani, 2009). Biopsy should be considered for those in whom more accurate fibrosis staging would impact treatment decisions. Individuals with clinically evident cirrhosis do not require additional staging (biopsy or noninvasive assessment).
Ongoing assessment of liver disease is especially important in patients for whom therapy has been deferred. In line with evidence-driven recommendations for treatment of nearly all HCV-infected patients, several factors must be taken into consideration if treatment deferral is entertained or mandated by lack of medication access. As noted, strong and accumulating evidence argue against deferral because of decreased all-cause morbidity and mortality, prevention of onward transmission, and quality-of-life improvements for patients treated regardless of baseline fibrosis. Additionally, successful HCV treatment may improve or prevent extraheptatic complications, including diabetes mellitus, cardiovascular disease, renal disease, and B-cell non-Hodgkin lymphoma (Torres, 2015); (Hsu, 2015); (Conjeevaram, 2011), which are not tied to fibrosis stage (Petta, 2016); (Allison, 2015). Deferral practices based on fibrosis stage alone are inadequate and shortsighted.
Fibrosis progression varies markedly between individuals based on host, environmental, and viral factors (Table 1); (Feld, 2006). Fibrosis may not progress linearly. Some individuals (often those aged >50 years) may progress slowly for many years followed by accelerated fibrosis progression. Others may never develop substantial liver fibrosis despite longstanding infection. The presence of existing fibrosis is a strong risk factor for future fibrosis progression. Fibrosis results from chronic hepatic necroinflammation; thus, a higher activity grade on liver biopsy and higher serum transaminase levels are associated with more rapid fibrosis progression (Ghany, 2003). However, even patients with a normal ALT level may develop substantial liver fibrosis over time (Pradat, 2002); (Nutt, 2000). The limitations of transient elastography and liver biopsy in ascertaining the progression of fibrosis must be recognized.
Host factors associated with more rapid fibrosis progression include male sex, longer duration of infection, and older age at the time of infection (Poynard, 2001). Many patients have concomitant nonalcoholic fatty liver disease. The presence of hepatic steatosis (with or without steatohepatitis) on liver biopsy, elevated body mass index, insulin resistance, and iron overload are associated with fibrosis progression (Konerman, 2014); (Everhart, 2009). Chronic alcohol use is an important risk factor because alcohol consumption has been associated with more rapid fibrosis progression (Feld, 2006). A safe amount of alcohol consumption has not been established. Cigarette smoking may also lead to more rapid fibrosis progression. For more counseling recommendations, see Testing and Linkage to Care.
Immunosuppression leads to more rapid fibrosis progression, particularly in the settings of HIV/HCV coinfection and solid organ transplantation (Konerman, 2014); (Berenguer, 2013); (Macias, 2009). Therefore, immunocompromised patients should be treated even if they have mild liver fibrosis at presentation.
HCV RNA level does not correlate with stage of disease (degree of inflammation or fibrosis). Available data suggest that fibrosis progression occurs most rapidly in patients with genotype 3 (Kanwal, 2014); (Bochud, 2009). Aside from coinfection with HBV or HIV, no other viral factors are consistently associated with disease progression.
Although an ideal interval for assessment has not been established, annual evaluation is appropriate to discuss modifiable risk factors and update testing for hepatic function and markers of disease progression. For all individuals with advanced fibrosis, liver cancer screening dictates a minimum of evaluation every 6 months.
Table. Factors Associated With Accelerated Fibrosis Progression
| Host | Viral |
|---|---|
|
Nonmodifiable
Modifiable
|
|
Overview of Cost, Reimbursement, and Cost-Effectiveness Considerations for Hepatitis C Treatment Regimens
This section summarizes the US payer system, explains the concepts of cost, price, cost-effectiveness, value, and affordability, and addresses the cost-effectiveness of HCV treatment. This section aims to be informational. As described, actual costs are rarely known. Accordingly, the HCV guidance does not currently utilize cost-effectiveness analysis to guide recommendations.
Drug Cost and Reimbursement
Many organizations are involved with hepatitis C drug distribution and each can impact costs as well as decisions about which regimens are reimbursed (US GAO, 2015); (US CBO, 2015). The roles these organizations have in determining the actual price paid for drugs and who has access to treatment include the following:
- Pharmaceutical companies determine the wholesale acquisition cost (WAC) of a drug (analogous to a sticker price). The company negotiates contracts with other organizations within the pharmaceutical supply chain that allow for rebates or discounts to decrease the actual price paid.
- Pharmacy benefit managers (PBMs) act as intermediaries between pharmaceutical companies and health insurance companies. They negotiate contracts that may include restrictions on the types of providers or patients who can be reimbursed for treatment. They might also offer exclusivity (restrictions on which medications can be prescribed) in exchange for lower negotiated prices, often provided in the form of WAC discounts.
- Private insurance companies often have separate pharmacy and medical budgets, and use PBMs or directly negotiate drug pricing with pharmaceutical companies. Insurance companies determine formulary placement, which impacts the choice of regimens and out-of-pocket expenses for patients. An insurance company can cover private, managed care Medicaid, and Medicare plans and have different formularies for each line of business.
- Medicaid is a heterogeneous consortium of insurance plans that includes fee-for-service and managed care options. Most plans negotiate rebates with pharmaceutical manufacturers (through PBMs or individually). For single-source drugs such as all-oral HCV treatments, Medicaid plans receive the lowest price offered to any other payer (outside of certain government agencies), and the minimum Medicaid drug rebate is 23.1% of the average manufacturer price (AMP). Differences in negotiated contracts between plans have led to Medicaid patients in different states having widely varied access to HCV therapy (Lo Re, 2016); (Barua, 2015); (Canary, 2015). State Medicaid programs have benefited from the Patient Protection and Affordable Care Act (ACA), although such benefits are mitigated in states that have opted out of expanding Medicaid coverage under the ACA. As the price of HCV therapies has decreased, states have loosened their Medicaid treatment restrictions with a growing number providing treatment to all infected persons. Many states, however, continue to restrict access to HCV treatment based on stage of liver fibrosis or history of recent drug use. Proposed rollbacks of Medicaid expansion implemented under the ACA threaten to reduce insurance coverage among HCV-infected people and could lead to new treatment restrictions.
- Medicare covers HCV drugs through part D benefits and is prohibited by law from directly negotiating drug prices. These drug plans are offered through PBMs or commercial health plans, which may negotiate discounts or rebates with pharmaceutical companies.
- The Veterans Health Administration receives mandated rebates through the Federal Supply Schedule program, which sets drug prices for several government agencies (including the Department of Veterans Affairs, federal prisons, and the Department of Defense) and typically receives substantial discounts over average wholesale price (AWP).
- State prisons and jails are usually excluded from Medicaid-related rebates and often do not have the negotiating leverage of larger organizations and, therefore, may pay higher prices than most other organizations.
- Specialty pharmacies receive dispensing fees and may receive additional payments from contracted insurance companies, PBMs, or pharmaceutical companies to provide services such as adherence support and/or management of adverse effects, and outcome measurements, such as early discontinuation rates and sustained virologic response rates.
- Patients incur costs (eg, copayment or coinsurance) determined by their pharmacy plan. Patient assistance programs offered by pharmaceutical companies or foundations can cover many of these out-of-pocket expenses or provide drugs at no cost to qualified patients who are unable to pay.
Except for mandated rebates, negotiated drug prices are considered confidential business contracts. Therefore, there is almost no transparency regarding the actual prices paid for hepatitis C drugs (Saag, 2015). However, the average negotiated discount of 22% in 2014 increased to 46% less than the WAC in 2015, implying that many payers are paying well below the WAC for HCV medications (Committee on Finance US Senate, 2016).
Cost-Effectiveness
Cost-effectiveness analysis (CEA) compares the relative costs and outcomes of 2 or more interventions. CEA explicitly recognizes budget limitations for healthcare spending and seeks to maximize public health benefits within those budgetary constraints. The core question that CEA addresses is whether to invest limited healthcare dollars in a new treatment/therapy or use that money to invest in another healthcare intervention that would provide better outcomes for the same monetary investment. The focus of CEA is, therefore, not simply cost or saving money but health benefits. It assumes that all available resources will be spent and provides a framework for prioritizing among available treatment options by formally assessing the comparative costs and health benefits accrued from a new treatment relative to current treatment.
The cost-effectiveness of a treatment is typically expressed as an incremental cost-effectiveness ratio (ICER).
| cost new treatment - cost current treatment |
| benefit new treatment - benefit current treatment |
Estimating and Interpreting the ICER
Estimating and interpreting the ICER requires answering 3 questions:
-
How much more money will be spent with the new treatment versus the old treatment?
The additional cost of new treatment includes that of new medications as well as the costs that will be avoided by preventing disease complications. Prevention of long-term complications is especially important when considering the cost-effectiveness of HCV treatments because the costs of the therapy are immediate, while those avoided by preventing advanced liver disease and other complications of chronic infection often accrue years in the future.
-
How much more benefit will occur with the new versus the old treatment?
Life expectancy is a valuable measure of benefit but considering only mortality benefits fails to recognize the value of treatments that improve quality of life. The quality-adjusted life-year (QALY) provides a measure that integrates both longevity and quality of life and is the preferred outcome for CEA.
-
How is the ICER to be interpreted?
The ideal CEA would list every possible healthcare intervention, its lifetime medical cost, and QALYs lived. Such a list would allow for perfect theoretical prioritization of spending to maximize QALY across the population. In reality, CEA compares the ICER for a specific treatment to a threshold value and rejects treatments with an ICER exceeding a particular threshold as not being cost-effective. The threshold value is referred to as the societal willingness-to-pay threshold. It is not meant to be a valuation of how much society is willing to pay to save a life. Rather, it is meant to reflect the average return in QALY expected if the available budget was not used to provide a new treatment but instead invested into the current healthcare system. In the United States, the willingness-to-pay threshold is typically considered to be $50,000 or $100,000 per QALY gained.
Affordability
An intervention that is cost-effective is not necessarily affordable. Affordability refers to whether a payer has sufficient resources in its annual budget to pay for a new therapy for all who might need or want it within that year. Several characteristics of CEA limit its ability to speak to the budgetary impact of interventions being implemented in the real world.
-
Perspective on cost
CEA seeks to inform decisions about how society should prioritize healthcare spending. As such, it typically assumes a societal perspective on costs and includes all costs from all payers, including out-of-pocket expenses for the patient. When making coverage decisions for therapy, however, an insurer considers only its own revenues and expenses.
-
Time horizon
From a societal perspective, CEA uses a lifetime time horizon, meaning it considers lifetime costs and benefits, including those that occur in the distant future. Business budget planning, however, typically assumes a 1-year to 5-year perspective. Savings that may accrue 30 years from now have no impact on spending decisions today because they have little bearing on the solvency of the current budget.
-
Weak association between willingness-to-pay and the real-world bottom line
Societal willingness-to-pay thresholds in CEAs are not based on actual budget calculations and have little relationship to a payer’s bottom line. Willingness-to-pay is meant to be an estimate of the opportunity cost of investing in a new therapy. In economics, opportunity cost refers to how else that money could have been spent and the benefits lost from not investing in that alternative (Wong, 2017a). When payers make a decision about coverage, the calculation is more straightforward and relates to the short-term cost of medications and the budgetary impact. Given the rapid development of new technologies and therapies, funding all of them (even if they all fell below the societal willingness-to-pay threshold) would likely lead to uncontrolled growth in demand and exceed the limited healthcare budget.
There is no formula that provides a good means of integrating the concerns of value and affordability. When new HCV therapies are deemed cost-effective, it indicates that these therapies provide good benefit for the resources invested and providing such therapy to more people would be a good long-term investment. Determining the total resources that can be spent on HCV treatment, however, depends on political and economic factors that are not captured by cost-effectiveness determinations.
Cost-Effectiveness of Current Direct-Acting Antiviral Regimens for Hepatitis C Treatment
Since the first direct-acting antivirals (DAAs) received US Food and Drug Administration approval in 2011, several cost-effectiveness investigations have compared DAA-based regimens to previous standard-of-care regimens to calculate ICERs. They have also investigated the cost-effectiveness of eliminating HCV treatment restrictions. Compared to interferon-based regimens, the ICER for DAAs has consistently been estimated at <$100,000 per QALY for all genotypes and fibrosis stages.
Several studies have compared DAA regimens against one another. In general, when given a choice between recommended HCV DAA regimens, the less costly regimen is preferred as a more efficient use of resources (even if it requires multiple tablet dosing). Because of the similar efficacy of most DAA regimens, cost becomes the critical factor driving relative cost-effectiveness. Studies have also estimated the cost-effectiveness of HCV treatment in special populations, including patients awaiting liver transplantation, HIV/HCV-coinfected patients, those with chronic kidney disease, persons who inject drugs, and adolescents—all with favorable ICERs. At this time, it is reasonable to conclude that DAA regimens provide good value for the resources invested.
Cost vs Affordability for HCV Treatment
Despite a growing body of evidence that HCV treatment is cost-effective and may even be cost saving over the long term in some cases, many US payers—especially those offering Medicaid insurance products—continue to limit access to HCV treatment. Access has improved as cost has decreased but limitations remain. Proposed reductions in healthcare spending for Medicaid would likely exacerbate the problem as the value of the HCV medications would remain unchanged but the resources available to provide them would shrink.
Cost-Effectiveness of Screening for HCV
Several cost-effectiveness studies demonstrate that routine, one-time testing for HCV among all adults in the US would likely identify a substantial number of cases of HCV that are currently being missed, and that doing so would be cost-effective. One study employed simulation modeling to compare several versions of routine guidance, including routine testing for adults over the ages of 40 years, 30 years, and 18 years and found that routine testing for all adults aged ≥18 years was cost-effective compared to risk-based screening, and potentially cost-saving compared to testing only those aged ≥30 years or aged ≥40 years (Barocas, 2018). The study further found that routine testing remained cost-effective unless HCV infection had no impact on healthcare utilization and no impact on quality of life. Another research group similarly found that routine testing of all adults aged ≥18 years is likely cost-effective compared to risk-based screening, so long as the prevalence of HCV among those born after 1965 exceeds 0.07% (Eckman, 2019). Notably, these studies reached similar conclusions despite being conducted entirely independently and employing different simulation modeling approaches. Further, a variety of studies have examined the cost-effectiveness of routine HCV testing in specific venues, including correctional settings (He, 2016), prenatal care settings (Chaillon, 2019); (Tasillo, 2019), substance use treatment centers (Schackman, 2018); (Schackman, 2015), and federally qualified health centers (Assoumou, 2018). All of them found that routine testing and treatment for HCV was cost-effective, even when linkage to HCV treatment after testing was poor, and even when the rate of HCV reinfection among injection drug users is common.
Generally, routine HCV testing is cost-effective because the incidence and prevalence of HCV remain high in people who inject drugs with a notable rising prevalence in young adults who may not readily report their stigmatized risk behaviors. Studies conducted in urban emergency departments in the US, for example, reveal that 15% to 25% of patients with previously unidentified HCV infection were born after 1965 and/or have no reported history of injection drug use and are, therefore, missed by even perfect implementation of risk-based screening (Schechter-Perkins, 2018); (Hsieh, 2016); (Lyons, 2016). Reinfection among those actively using drugs is common but because screening is a low-cost intervention, and therapy is both highly effective and cost-effective, routine testing provides good economic value (ie, cost-effective) even when many people need to be tested and treated more than once during their lifetime.
Conclusions
Many studies have demonstrated the economic value of HCV screening (Chaillon, 2019); (Eckman, 2019); (Tasillo, 2019); (Assoumou, 2018); (Barocas, 2018); (Schackman, 2018); (Schechter-Perkins, 2018); (Lyons, 2016); (Hsieh, 2016); (Schackman, 2015) and treatment (Goel, 2018); (Chhatwal, 2017); (He, 2017); (Chahal, 2016); (Chhatwal, 2015); (Chidi, 2016); (Martin, 2016a); (Linas, 2015); (Najafzadeh, 2015); (Rein, 2015); (Tice, 2015); (Younossi, 2015a) and made it clear that HCV screening and therapy are cost-effective. In response, in 2020, both the US Centers for Disease Control (CDC) and Prevention and the US Preventive Service Task Force (USPSTF) recommended routine, one-time HCV testing for all US asymptomatic adults aged 18 to 79 without known liver disease (Owens, 2020); (Schillie, 2020). A US study found reductions in measures of health care utilization (i.e. liver-related emergency department visits, liver-related hospitalizations, and all-cause hospitalizations) among cases who achieved SVR after DAA therapy compared to matched controls (Gordon, 2022). The high cost of HCV medications and the high prevalence of disease have led to limited access for some patients. The issue is complex. Although the wholesale acquisition costs of HCV drugs often make treatment appear unaffordable, the reality is that insurers, PBMs, and government agencies negotiate pricing, and few actually pay this much-publicized price. Negotiated pricing and cost structure for pharmaceutical products in the US, however, are not transparent. Thus, it is difficult to estimate the true budgetary impact of providing HCV drugs. Competition and negotiated pricing have reduced prices substantially but cost continues to limit the public health impact of DAA therapies. Insurers, government, and pharmaceutical companies should work together to bring medication prices to the point where all persons in need of treatment are able to afford and readily access it. Only 3 US states and only 24% of high-income countries are on target to meet the WHO 2030 hepatitis C elimination targets (Gamkrelidze, 2021); (Sulkowski 2021).
Monitoring Patients Who Are Starting HCV Treatment, Are on Treatment, or Have Completed Therapy
This section provides guidance on monitoring patients with chronic hepatitis C virus (HCV) infection who are starting direct-acting antiviral (DAA) treatment, are on treatment, or have completed therapy. It is divided into 4 parts: pretreatment and on-treatment monitoring (including patients with incomplete adherence); posttreatment follow-up for persons in whom treatment failed to clear the virus; posttreatment follow-up for those who achieve a sustained virologic response (SVR; virologic cure); and additional considerations if treatment includes ribavirin.
Pretreatment and On-Treatment Monitoring
Recommended Assessments Prior to Starting DAA Therapy |
|
|---|---|
| RECOMMENDED |
RATING |
|
Staging of hepatic fibrosis is essential prior to HCV treatment (see Testing and Linkage to Care and see When and in Whom to Treat).
Assessment of potential drug-drug interactions with concomitant medications is recommended prior to starting DAA therapy and, when possible, an interacting co-medication should be stopped or switched to an alternative with less risk for potential interaction during HCV treatment. (See Table of Drug Interactions with Direct-Acting Antivirals and Selected Concomitant Medications below or use an online resource such as University of Liverpool interaction checker.)
Patients should be educated about the proper administration of DAA medications (eg, dose, frequency of medicines, food effects, missed doses, adverse events, etc), the crucial importance of adherence, and the need to inform the healthcare provider about any changes to their medication regimen.
|
I, C |
| The safety of ribavirin-free DAA regimens in humans has not been established during pregnancy and for nursing mothers, so counseling should be offered to women of childbearing age before beginning HCV treatment. (See ribavirin pregnancy recommendations below.) | I, C |
| All patients initiating DAA therapy should be assessed for active hepatitis B virus (HBV) coinfection with HBV surface antigen (HBsAg) testing, and for evidence of prior infection with HBV core antibody (anti-HBc) and HBV surface antibody (anti-HBs) testing. | IIa, B |
| Patients found or known to be HBsAg-positive should be assessed for whether their HBV DNA level meets AASLD criteria for HBV treatment and initiation of antiviral therapy for HBV. | Strong, Moderatea |
| All patients should be assessed for HIV coinfection prior to initiating DAA therapy. | IIa, B |
| Testing for the presence of resistance-associated substitutions (RASs) prior to starting treatment should be performed as recommended in the Initial Treatment and the Retreatment sections. Additional information about RAS testing can be found in the HCV Resistance Primer. | IIb, B |
Patients scheduled to receive an HCV NS3 protease inhibitor (ie, grazoprevir, voxilaprevir, glecaprevir) should be assessed for a history of decompensated liver disease and liver disease severity using the Child-Turcotte-Pugh (CTP) score (see third-party calculator).
|
I, A |
| a Unlike the AASLD/IDSA HCV guidance, the AASLD guidelines for treatment of chronic hepatitis B uses the GRADE system to rate recommendations; please see that document for further information about this rating system. | |
Recommended Monitoring During Antiviral Therapy |
|
|---|---|
| RECOMMENDED |
RATING |
| Clinic visits or telephone contact are recommended as clinically indicated during treatment to ensure medication adherence and monitor for adverse events and potential drug-drug interactions (see table of Drug Interactions with Direct-Acting Antivirals and Selected Concomitant Medications below), especially with newly prescribed medications. | I, B |
| Inform patients taking diabetes medication of the potential for symptomatic hypoglycemia. On-treatment and posttreatment monitoring for hypoglycemia is recommended. | I, C |
| Inform patients taking warfarin of the potential for changes in their anticoagulation status. On-treatment and posttreatment INR monitoring for subtherapeutic anticoagulation is recommended. | I, C |
|
Patients receiving elbasvir/grazoprevir should be monitored with a hepatic function panel at 8 weeks and again at 12 weeks if receiving 16 weeks of treatment. |
I, B |
|
A ≥10-fold increase in ALT values from baseline at any time during treatment should prompt discontinuation of DAA therapy (especially with signs or symptoms of liver inflammation or increasing conjugated bilirubin, alkaline phosphatase, or INR).
An increase in ALT <10-fold from baseline that is accompanied by any weakness, nausea, vomiting, jaundice, or significantly increased bilirubin, alkaline phosphatase, or INR should also prompt discontinuation of DAA therapy. Asymptomatic increases in ALT <10-fold from baseline should be closely monitored with repeat testing at 2-week intervals. If levels remain persistently elevated, consideration should be given to discontinuation of DAA therapy. |
I, B |
|
Quantitative HCV viral load testing is recommended 12 or more weeks after completion of therapy to document sustained virologic response (SVR), which is consistent with cure of chronic HCV infection. |
I, B |
For HBsAg-positive patients not already receiving HBV suppressive therapy because their baseline HBV DNA level does not meet treatment criteria, one of two approaches may be taken:
|
IIa, B |
The recommended pretreatment testing assumes that a decision to treat with antiviral medications has already been made and that the testing involved in deciding to treat—including testing for HCV genotype and assessment of hepatic fibrosis—has already been completed (see When and in Whom to Initiate HCV Therapy).
Prior to starting treatment, patients should be evaluated for potential drug-drug interactions with selected antiviral medications by consulting the prescribing information and using other resources (eg, http://www.hep-druginteractions.org). The table below lists known drug-drug interactions between HCV DAAs and selected medications.
Table. Drug Interactions with Direct-Acting Antivirals and Selected Concomitant Medications
| Concomitant Medications | SOF/VEL | GLE/PIB | SOF/VEL/VOX | LDV/SOF | EBR/GZR |
|---|---|---|---|---|---|
| Acid-reducing agents |
Antacids H2RA PPI |
H2RA PPI |
Antacids H2RA PPI |
Antacids H2RA PPI |
|
| Alpha-1 blockers |
Prazosin Silodosin |
Prazosin Silodosin |
Prazosin Silodosin |
Silodosin |
Prazosin Silodosin |
| Analgesics | Metamizole |
Alfentanil |
Metamizole |
Fentanyl |
|
| Antiarrhythmics |
Amiodarone Dronedarone |
Amiodarone |
Amiodarone Dronedarone |
Amiodarone Dronedarone |
Amiodarone Dronedarone Quinidine |
|
Digoxin Quinidine |
Digoxin Quinidine |
Digoxin Quinidine |
|||
| Anticoagulant and antiplatelet agents |
Apixaban Dabigatran Edoxaban Rivaroxaban Ticagrelor Warfarin |
Dabigatran |
Dabigatran Edoxaban |
Apixaban Dabigatran Edoxaban Rivaroxaban Ticagrelor Warfarin |
Apixaban Dabigatran Edoxaban Rivaroxaban Ticagrelor Warfarin |
|
Apixaban Edoxaban Rivaroxaban Ticagrelor Warfarin |
Apixaban Rivaroxaban Ticagrelor Warfarin |
||||
| Anticonvulsants and barbiturates |
Amobarbital Carbamazepine Eslicarbazine Oxcarbazepine Phenobarbital Phenytoin Primidone |
Amobarbital Carbamazepine Eslicarbazine Oxcarbazepine Phenobarbital Phenytoin Primidone |
Amobarbital |
Amobarbital Carbamazepine Oxcarbazepine Phenobarbital Phenytoin Primidone |
Amobarbital Carbamazepine Eslicarbazine Oxcarbazepine Phenobarbital Phenytoin Primidone |
| Rufinamide | Rufinamide | Rufinamide | Rufinamide Zonisamide | Rufinamide | |
| Antihypertensives | Diltiazem | Aliskiren |
Aliskiren Enalapril Irbesartan Isradipine Non-DHP CCB Olmesartan Telmisartan Valsartan |
Aliskiren Amlodipine Diltiazem Eplerenone Felodipine Irbesartan Isradipine |
Eplerenone Felodipine Isradipine |
|
Enalapril Eplerenone Irbesartan Isradipine Non-DHP CCB Olmesartan Telmisartan |
|||||
| Antimycobacterials |
Rifabutin |
Rifabutin |
Rifabutin |
Bedaquiline |
Rifabutin |
|
Bedaquiline |
Rifabutin |
Bedaquiline |
|||
| Antipsychotics – first generation | Pimozide | Pimozide | Pimozide | ||
|
Droperidol Thioridazine |
|||||
| Antipsychotics – second generation |
Aripiprazole Clozapine Paliperidone Quetiapine |
Paliperidone | Paliperidone |
Aripiprazole Quetiapine |
|
| Antiretrovirals | See HIV/HCV Coinfection Section | ||||
| Azole antifungals |
Ketoconazole Posaconazole |
Ketoconazole | |||
| Benzodiazepines | Midazolam | ||||
|
Bronchodilators |
Theophylline | ||||
| Buprenorphine/ naloxone | |||||
| Calcineurin inhibitors |
Cyclosporine |
Cyclosporine | Cyclosporine | ||
| Tacrolimus | Tacrolimus | ||||
| Cancer Therapies |
Acalabrutinib |
Acalabrutinib |
Acalabrutinib |
Acalabrutinib |
Acalabrutinib |
|
Vinblastine |
Imatinib |
||||
| Cholesterol-lowering agents |
Atorvastatin Fluvastatin Lovastatin Pitavastatin Rosuvastatin Simvastatin |
Atorvastatin Lovastatin Simvastatin |
Atorvastatin Fluvastatin Lovastatin Pitavastatin Rosuvastatin Simvastatin |
Rosuvastatin |
Atorvastatin Fluvastatin Gemfibrozil Lovastatin Rosuvastatin Simvastatin |
|
Ezetimibe Fluvastatin Gemfibrozil Pitavastatin Pravastatin Rosuvastatin |
Ezetimibe |
Atorvastatin Fluvastatin Lovastatin Pitavastatin Pravastatin Simvastatin |
|||
| Cisapride | |||||
| COVID-19 antivirals |
Molnupiravir |
Molnupiravir |
Molnupiravir |
Molnupiravir |
Molnupiravir |
|
Nirmatrelvir/ |
Nirmatrelvir/ |
Nirmatrelvir/ |
|||
| Ergot derivatives | |||||
| Ethinyl estradiol containing products | |||||
| Glucocorticoids | Dexamethasone | Dexamethasone | Dexamethasone | ||
| Heart failure agents | Bosentan | Bosentan | Bosentan | Bosentan | |
| Ambrisentan | Ambrisentan | Ambrisentan | |||
| Herbals | St. John’s wort | St. John’s wort | St. John’s wort | St. John’s wort | St. John’s wort |
| Loop diuretics | |||||
| Macrolide antimicrobials | Troleandomycin |
Erythromycin Telithromycin |
Erythromycin Telithromycin |
Telithromycin | Telithromycin |
| Troleandomycin | Troleandomycin | Troleandomycin | |||
| Phosphodiesterase-5 inhibitors | |||||
| Recreational Drugs |
Carfentanil |
Carfentanil |
|||
|
H2RA=Histamine H2 Antagonist; PPI=proton pump inhibitor; DHP CCB=dihydropyridine calcium channel blocker; Non-DHP CCB=non dihydropyridine calcium channel blocker. Green indicates coadministration is safe; yellow indicates a dose change or additional monitoring is warranted; and red indicates the combination should be avoided. Specific concomitant medications or medication classes with actual or theoretical potential for interaction are listed in the box. |
|||||
The education of patients and caregivers about potential adverse effects of DAA therapy and their management is an integral component of treatment and is important for a successful outcome in all patient populations. During DAA treatment, individuals should be followed at clinically appropriate intervals to ensure medication adherence, assess adverse events and potential drug-drug interactions, and monitor blood test results necessary for patient safety. This includes on-treatment and posttreatment monitoring for hypoglycemia or subtherapeutic INR levels among patients taking diabetes medicines or warfarin, respectively. Real-world data indicate an association between DAA therapy and related changes in hepatic function and alterations in dose-response relationships with these medications (Drazilova, 2018); (Abdel Alem, 2017); (Rindone, 2017); (Pavone, 2016); (DeCarolis, 2016); (Soriano, 2016). Inform patients on these medications about the potential for these developments; make dose adjustments as needed. The frequency and type of contact (eg, clinic visit, phone call, etc) are variable but need to be sufficient to assess patient safety and response to treatment, as outlined above.
Routine testing for HCV RNA during treatment is not recommended unless the ALT level fails to decline (when elevated) or there are concerns regarding patient adherence with DAA treatment. There are no data to support stopping treatment based on detectable HCV RNA during the first 4 weeks of treatment, or that detectable HCV RNA at this time point signifies medication nonadherence.
It is essential to test for HCV RNA 12 weeks (or longer) after treatment completion. Undetectable or unquantifiable HCV RNA 12 weeks or longer after treatment completion is defined as a sustained virologic response (SVR), which is consistent with cure of chronic HCV infection. Virologic relapse is rare 12 weeks or longer after treatment completion (Sarrazin, 2017); (Simmons, 2016). Nevertheless, repeat quantitative HCV RNA testing can be considered at 24 or more weeks after completing treatment for patients in whom ALT increases to above the upper limit of normal.
During clinical trials with elbasvir/grazoprevir, with or without ribavirin, 1% of participants experienced ALT elevations from normal levels to >5 times the upper limit of normal, generally at or after treatment week 8. ALT elevations were typically asymptomatic, and most resolved with ongoing therapy or completion of therapy. Higher rates of late ALT elevations occurred in females, those of Asian descent, and patients aged ≥65 years. Hepatic laboratory testing should be performed prior to therapy, at treatment week 8, and as clinically indicated. For patients receiving 16 weeks of therapy, additional hepatic laboratory testing should be performed at treatment week 12 (Zepatier package insert, 2019).
One recent cohort study of 18,498 initiators of PI-based DAA therapy (paritaprevir/ritonavir/ombitasvir +/- dasabuvir, elbasvir/grazoprevir, glecaprevir/pibrentasvir) matched 1:1 on propensity score to non-PI-based DAA initiators (ledipasvir/sofosbuvir, sofosbuvir/velpatasvir) was conducted within the US Veterans Health Administration from 2014-2019 (Torgersen, 2021). During exposure to DAA therapy, the study determined incident development of: 1) ALT >200 U/L, 2) severe hepatic dysfunction (defined by coagulopathy with hyperbilirubinemia), and 3) hepatic decompensation, according to baseline FIB-4 score (≤3.25; >3.25). The analysis found that the risk of incident ALT elevations was increased among PI-based DAA initiators in both FIB-4 groups, but the risk of severe hepatic dysfunction or hepatic decompensation did not differ between PI and non-PI-based DAA initiators in either FIB-4 group.
Patients being treated with amiodarone should not receive sofosbuvir-based regimens due to risk of life-threatening arrhythmias. Because of its long half-life, it is advised that persons should be off amiodarone for at least 6 months before initiating sofosbuvir. If the decision is made to start sofosbuvir in this setting, continued vigilance for bradycardia should be exercised.
Simplified HCV Treatment for Treatment-Naïve Adults
Recent data from a global sample of patients undergoing antiviral treatment for chronic HCV infection suggested that a minimal monitoring approach was safe and achieved SVR at a rate comparable to that with standard monitoring. This minimal monitoring approach was examined in a phase 4, open label, single-arm trial that enrolled 400 treatment-naïve patients 18 years or older with HCV RNA >1,000 IU/mL from Brazil, South Africa, Thailand, Uganda, and the USA with capped inclusion of compensated cirrhosis and HIV/HCV coinfection (Solomon, 2022). Pregnancy, breastfeeding, or evidence of chronic hepatitis B virus (HBV) infection (HBsAg-positive) were exclusion criteria; however, participants with resolved HBV infection (hepatitis B core total antibody [anti-HBc] with or without positive hepatitis B surface antibodies [anti-HBs]) were eligible. Patients initiated treatment with fixed dose sofosbuvir (400 mg)/velpatasvir (100 mg) once daily for 12 weeks. Minimal monitoring involved: 1) no pre-treatment genotyping; 2) dispensing the entire treatment course (84 tablets) at entry; 3) no scheduled visits or laboratory monitoring; and 4) remote contact at week 4 to assess DAA adherence and at week 22 to schedule SVR assessment at week 24.
Of the 400 participants, 399 initiated sofosbuvir/velpatasvir treatment. At entry, 166 (42%) were living with HIV, 34 (9%) had compensated cirrhosis, and 121 (32%) of 374 with HBV panel (HBsAg, anti-HBc, anti-HBs) available had evidence of resolved HBV infection. Overall, 379 of the 399 who initiated treatment achieved SVR (95.0%; 95% CI, 92.4-96.7%). A total of 14 (4%) of 397 participants reported serious adverse events between treatment initiation and week 28, but none were treatment-related or led to treatment discontinuation or death. Because of the possible risk for HBV reactivation that could be fulminant, these HBV-HCV co-infected patients should be excluded.
Given the findings of this minimal monitoring study, simplified HCV treatment approaches are available for HCV treatment-naïve adults without cirrhosis (click here) and for HCV treatment-naïve adults with compensated cirrhosis (click here).
Pregnancy and Nursing Mothers
Few adequate and well-controlled human studies are available to establish whether DAAs pose a risk to pregnancy outcomes or whether DAAs and their metabolites are present in breastmilk. An open-label, phase 1 study of HIV-negative pregnant women with chronic genotype 1 infection evaluated a 12-week course of ledipasvir/sofosbuvir initiated between 23 to 24 weeks of gestation (Chappell, 2019). Among 7 evaluable patients, all achieved SVR12; adverse events related ledipasvir/sofosbuvir were ≤ grade 2. All 7 participants delivered at term with undetectable HCV viral loads at delivery. One-year follow-up of the infants is ongoing.
Given the dearth of data on this topic, clinicians should discuss with female patients that DAAs should be used during pregnancy only if the potential benefit of DAA therapy justifies the potential risk of harm to the fetus. The health benefits of DAA therapy for nursing mothers should be weighed against the health benefits of breast feeding and the possible adverse effects of the DAA regimen on the breastfed child. Given the relatively short duration of treatment and the availability of ribavirin-free regimens in most patients, the potential risk of harms and benefits of delaying pregnancy until HCV DAA therapy is completed should be considered. For additional information about HCV and pregnancy, click here.
Reactivation of Hepatitis B Virus Infection
Cases of hepatitis B virus (HBV) reactivation, occasionally fulminant, during or after DAA therapy have been reported in HBV/HCV coinfected patients who were not receiving HBV suppressive therapy (Mücke, 2018); (Bersoff-Matcha, 2017); (Chen, 2017). Therefore, all patients initiating DAA therapy should be assessed for HBV coinfection with HBsAg testing, and for evidence of prior infection with anti-HBc and anti-HBs testing. HBV vaccination is recommended for all susceptible individuals. Testing for HBV DNA should be performed prior to DAA therapy in patients who are HBsAg positive. HBsAg-positivity does not represent a contraindication to DAA therapy. Patients meeting criteria for treatment of active HBV infection should be started on HBV therapy at the same time or before DAA therapy is initiated (Terrault, 2018).
Patients with a low or undetectable HBV DNA level can either receive prophylactic HBV treatment for the duration of DAA treatment until assessment for SVR12 or be monitored at regular intervals (usually not more frequently than every 4 weeks) for HBV reactivation with HBV DNA testing. If monitoring is elected, HBV treatment should be started if the HBV DNA level increases >10-fold or is >1000 IU/mL in a patient with undetectable or unquantifiable HBV DNA prior to DAA treatment. There are insufficient data to provide clear recommendations for the monitoring of HBV DNA among patients testing positive either for anti-HBc alone (isolated anti-HBc) or for both anti-HBc and anti-HBs (resolved infection). However, the possibility of HBV reactivation should be considered in these patients in the event of an unexplained increase in liver aminotransferase levels during and/or after completion of DAA therapy.
Posttreatment Follow-Up for Patients in Whom Treatment Failed
Recommended Monitoring for Patients in Whom Treatment Failed to Achieve a Sustained Virologic Response |
|
|---|---|
| RECOMMENDED |
RATING |
| Retreatment for chronic HCV is recommended utilizing the regimens recommended in the Retreatment section. | I, C |
| Disease progression assessment every 6 to 12 months with a hepatic function panel, complete blood count (CBC), and international normalized ratio (INR) is recommended if patients are not retreated or fail a second or third DAA treatment course. | I, C |
| Surveillance for hepatocellular carcinoma with liver ultrasound examination, with or without alpha fetoprotein (AFP), every 6 months is recommended for patients with cirrhosisa in accordance with the AASLD guidance on the diagnosis, staging, and management of hepatocellular carcinoma. | Low, Conditionalb |
| For patients with cirrhosis, endoscopic surveillance for varices should be performed in accordance with the AASLD guidance on portal hypertension bleeding in cirrhosis. | Guidanceb |
|
a For decompensated cirrhosis, please refer to the appropriate section. b Unlike the AASLD/IDSA HCV guidance, the AASLD guidelines use the GRADE system to rate recommendations; please see that document for further information about this rating system. |
|
The Following Monitoring Is Not Recommended During or After Therapy |
|
|---|---|
| NOT RECOMMENDED |
RATING |
| Monitoring for HCV drug resistance-associated substitutions (RASs) during or after therapy is not recommended unless retreatment will be performed. RAS testing is recommended in advance of retreatment therapy. See the Retreatment section for recommendations regarding RAS testing prior to retreatment. Additional information about RAS testing can be found in the HCV Resistance Primer. | IIb, C |
Patients who do not achieve SVR retain the possibility of continued liver injury, progression of hepatic fibrosis, and the potential to transmit HCV infection to others. Such patients should be considered for retreatment per the Retreatment of Persons in Whom Prior Therapy Has Failed section.
Given that persons in whom treatment failed remain at risk for ongoing liver injury and liver fibrosis progression (Dienstag, 2011), these patients should be monitored for signs and symptoms of decompensated cirrhosis. Patients in whom antiviral therapy failed may harbor viruses that are resistant to one or more of the antivirals at the time of virologic breakthrough (Lawitz, 2014a); (Schneider, 2014). There is no evidence to date, however, that the presence of resistance-associated substitutions (RASs) results in more progressive liver injury than would have occurred if the patient did not have resistant viruses. Additional information about RASs and RAS testing can be found in the HCV Resistance Primer section. If there remains uncertainty regarding the applicability of RAS testing, consultation with an expert regarding the treatment of HCV infection may be useful.
Posttreatment Follow-Up for Patients Who Achieved a Sustained Virologic Response
Recommended Follow-Up for Patients Who Achieved a Sustained Virologic Response (SVR) |
|
|---|---|
| RECOMMENDED |
RATING |
| For noncirrhotic patients, recommended follow-up is the same as if they were never infected with HCV. | I, B |
| Assessment for HCV recurrence is recommended only if the patient develops unexplained hepatic dysfunction, or annual assessment if the patient has ongoing risk factors for HCV infection. In such cases, a quantitative HCV-RNA test rather than an HCV-antibody test is recommended to assess for HCV recurrence. | I, A |
| Surveillance for hepatocellular carcinoma is recommended for patients with cirrhosis,a in accordance with the AASLD guidance on the diagnosis, staging, and management of hepatocellular carcinoma. | Strong, Moderateb |
| For cirrhotic patients, upper endoscopic surveillance is recommended in accordance with the AASLD guidance on portal hypertension bleeding in cirrhosis. | Guidanceb |
| Assessment for other causes of liver disease is recommended for patients who develop persistently abnormal liver tests after achieving SVR. | I, C |
|
a For decompensated cirrhosis, please refer to the appropriate section. b Unlike the AASLD/IDSA HCV guidance, the AASLD guidelines use the GRADE system to rate recommendations; please see that document for further information about this rating system. |
|
Patients with undetectable serum HCV RNA, as assessed by a sensitive polymerase chain reaction (PCR) assay, ≥12 weeks after treatment completion are deemed to have achieved SVR (ie, cure). The likelihood of achieving SVR with DAA therapy among adherent, immunologically competent, treatment-naive patients with compensated liver disease generally exceeds 95%. Among patients who achieved SVR with peginterferon/ribavirin treatment, more than 99% have remained free of HCV infection when followed for 5 years after treatment completion (Manns, 2013). Thus, achieving SVR is considered a virologic cure of HCV infection. SVR typically aborts progression of liver injury with regression of liver fibrosis in most (but not all) treated patients (Morgan, 2013); (Morisco, 2013); (Morgan, 2010); (Singal, 2010); (George, 2009). Liver fibrosis and liver function test results improve in most patients who achieve SVR (Morgan, 2013); (Morisco, 2013); (Morgan, 2010); (Singal, 2010); (George, 2009). Because of lack of progression, noncirrhotic patients who achieve SVR should receive standard medical care that is recommended for patients who were never infected with HCV unless they remain at risk for non-HCV–related liver disease, such as nonalcoholic fatty liver disease or alcoholic liver disease.
Among cirrhotic patients who achieve SVR, decompensated liver disease (with the exception of hepatocellular carcinoma [HCC]) rarely develops during follow-up and overall survival is prolonged (Morgan, 2013); (Morisco, 2013); (Morgan, 2010); (Singal, 2010); (George, 2009). Bleeding from esophageal varices is rare after SVR (Morgan, 2013); (Morisco, 2013); (Morgan, 2010); (Singal, 2010); (George, 2009). Cirrhotic patients should undergo surveillance endoscopy every 2 years if known to have small varices and every 3 years in the absence of known varices in accordance with AASLD guidance on portal hypertension bleeding (Garcia-Tsao, 2017).
Importantly, cirrhotic patients remain at risk for developing HCC and should, therefore, undergo surveillance for HCC every 6 months utilizing ultrasound (with or without AFP testing) despite the lowered risk that results after viral eradication (Marrero, 2018). Although multiple studies of cirrhotic patients who achieved SVR with peginterferon/ribavirin reported a reduction in the risk of developing HCC (Morgan, 2013); (Morisco, 2013); (Morgan, 2010); (Singal, 2010); (George, 2009) and a meta-analysis of persons achieving SVR with DAAs found that the HCC risk did not exceed that seen in patients who experienced SVR with interferon-based treatment after adjustment for baseline risk factors for HCC (Waziry, 2017b), one report found a higher than expected frequency of HCC in patients with HCV-related cirrhosis despite successful DAA treatment (Reig, 2016). However, a prospective observational study of 3045 cirrhotic patients found an adjusted hazard ratio for HCC of 0.57 (95% CI 0.40 to 0.81) following DAA-based therapy, implying a 43% reduction in HCC incidence (Carrat, 2019).
Bleeding from esophageal varices is uncommon after SVR (Morgan, 2013); (Morisco, 2013); (Morgan, 2010); (Singal, 2010); (George, 2009). Nevertheless, patients with compensated cirrhosis who achieve SVR should continue to receive endoscopic surveillance for esophageal varices, in accordance with the AASLD guidance on portal hypertension bleeding (Garcia-Tsao, 2017). Current AASLD recommendations for patients with compensated cirrhosis without known varices is surveillance endoscopy every 2 years if there is evidence of ongoing liver injury from associated conditions, such as obesity or alcohol use, and every 3 years if liver injury is quiescent, such as after alcohol abstinence. Patients with compensated cirrhosis and known varices should undergo surveillance endoscopy annually if there is evidence of ongoing liver injury from associated conditions, such as obesity or alcohol use, and every 2 years if liver injury is quiescent, such as after alcohol abstinence.
Patients in whom SVR is achieved but who have another potential cause of liver disease (eg, excessive alcohol use, metabolic syndrome with or without proven fatty liver disease, or iron overload) remain at risk for hepatic fibrosis progression. It is recommended that such patients be educated about the risk of liver disease and monitored for liver disease progression with periodic physical examination, blood tests, and potentially, tests for liver fibrosis by a liver disease specialist.
Patients who achieve SVR can have HCV recurrence due to reinfection or late relapse (Sarrazin, 2017); (Simmons, 2016). A systematic review suggests 5-year recurrence risks of 1%, 11%, and 15% in low-risk HCV monoinfected, high-risk HCV monoinfected (ie, people who currently or formerly injected drugs, imprisonment, or men who have sex with men [MSM]), and HIV/HCV coinfected patients, respectively (Simmons, 2016). At least annual testing for HCV reinfection among patients with ongoing risk for HCV infection (eg, injection drug use or high-risk sexual exposure) is recommended. A flare in liver aminotransferase levels should prompt immediate evaluation for HCV reinfection (see Management of Acute HCV Infection). Because HCV antibody remains positive in most patients after achieving SVR, testing for HCV recurrence using an assay that detects HCV RNA (ie, quantitative HCV-RNA test) is recommended.
Acute liver injury is common among patients receiving chemotherapy or immunosuppressive agents. Testing for hepatitis viruses should be included in the laboratory assessment of the cause of acute liver injury in these patients. Approximately 23% of patients with active HCV infection—especially those with a hematologic malignancy—experience a flare in their HCV RNA level (>10-fold) during chemotherapy. An ALT level increase is less common and clinical symptoms of hepatitis are uncommon (Torres, 2018). Among patients who have recovered from HCV infection, either spontaneously or with DAA treatment, reactivation of HCV infection (ie, detectable HCV RNA) during chemotherapy is distinctly uncommon and is not anticipated to occur since there is no residual reservoir for the virus. Thus, in this latter group, routine testing for HCV RNA during immunosuppressive treatment or prophylactic administration of antivirals during immunosuppressive treatment is not recommended.
Additional Considerations If Treatment Includes Ribavirin
Ribavirin causes hemolysis. Patients receiving ribavirin should have hemoglobin levels checked during treatment, often after 2 weeks, and the ribavirin dose reduced if the patient develops significant anemia, often defined as hemoglobin <10 g/dL.
Ribavirin causes fetal death and fetal abnormalities in animals. Ribavirin should not be administered to pregnant women or to women who might become pregnant during or for 6 months after completing ribavirin treatment. Similarly, ribavirin may cause birth defects in offspring of women whose partner was receiving ribavirin when the woman became pregnant. In the very rare instances when ribavirin is used, it is imperative for persons of childbearing potential to use at least 2 reliable forms of effective contraception during treatment and for a period of 6 months thereafter. It is recommended that the healthcare practitioner document the discussion of the potential teratogenic effects of ribavirin in the patient’s medical record.
Incomplete Adherence
There are minimal data regarding the outcome of patients who have incomplete adherence to DAA therapy or the threshold level of adherence below which the incidence of SVR12 is significantly reduced. Missing doses of DAAs is relatively common. A secondary analysis of data from electronic blister packs that recorded the date and time of each dose among 103 participants in a velpatasvir/sofosbuvir clinical trial demonstrated that approximately one-third (32%) of study participants had <90% adherence (ie, nonadherence) (Cunningham, 2018). The most common episodes of nonadherence lasted 1–2 days (61% of episodes); 11% of episodes lasted ≥7 days. Despite the nonadherence, SVR12 was 94% among both DAA adherent (≥90% of doses) and nonadherent (<90% of doses) patients. Longer durations of missed treatment, however, may affect SVR. A study of patients receiving DAA treatment found that only 50% (2/4) patients with F0-F3 disease who took <4 weeks of their course of therapy experienced SVR, compared with an SVR of 99% (109/110) for those who received ≥4 weeks of therapy. Among patients with cirrhosis, a lower SVR12 rate was observed in those who took <8 weeks of therapy compared with participants who took ≥8 weeks (83% [25/30] vs 95% [209/221]) (Fabbiani, 2021).
There are few data on which to base recommendations regarding how to manage patients who have discontinued DAAs for several days to weeks. The recommendations shown in Figure 1 are applicable to treatment-naive patients with acute or chronic HCV, without cirrhosis or with compensated cirrhosis, receiving either glecaprevir/pibrentasvir or sofosbuvir/velpatasvir. These recommendations are based on the opinion of the AASLD-IDSA HCV Treatment Guidance Panel.
Patients with prior DAA treatment, or receiving other DAA treatment regimens, or other populations (eg, patients who are posttransplant or have decompensated cirrhosis) should be managed in consultation with an expert. All patients with incomplete adherence should be asked about factors contributing to adherence or nonadherence, and counseled regarding the importance of adherence. In general, the panel considers a treatment interruption of <7 days unlikely to impact SVR12, based on adherence and outcome data from the SIMPLIFY study (Cunningham, 2018).
Click the graphic to enlarge Figure 1.
HCV Resistance Primer
Introduction
Understanding principles of the emergence of drug-resistant viruses is critical when using targeted antiviral therapies. The best example of these principles can be gleaned from the study of HIV. Like HIV, HCV is an approximately 9.5 kilobase RNA virus that replicates very rapidly (billions of viruses daily). The production of each new virus is performed by an enzyme that results in 1 to 3 errors per replication cycle, on average. Many of these errors either have no effect on the progeny virus product or result in progeny viruses that are nonreplication competent (i.e., dead viruses). For some newly produced viruses, however, the transcription errors result in changes in critical coding regions that may, by chance, change the susceptibility of the virus to 1 or more drugs used to treat the virus. The emergence of such drug-resistant viruses most often occurs when drug levels are subtherapeutic, thereby creating selective pressure for the resistant viruses to emerge as the dominant species. These newly formed resistant viruses have a selective growth advantage that allows them to replicate in the presence of antiviral drugs. In a subset of patients with chronic HCV infection, viral variants harboring substitutions associated with resistance to HCV directing-acting antivirals (DAAs) are detectable prior to antiviral therapy and, particularly in the case of NS5A inhibitor-containing regimens, may negatively impact treatment response. These substitutions often are referred to as baseline resistance-associated substitutions (RASs).
In the case of HCV DAAs, resistant viruses are also selected for and/or enriched in patients for whom a DAA regimen fails. These viruses contain substitutions that are designated as treatment-emergent (or treatment-selected) RASs. NS5A and NS3 RASs are frequently selected in patients with failure of NS5A or NS3 inhibitor-containing regimens, respectively. In contrast, NS5B nucleotide RASs are rarely detected (1% of failures) even after exposure to a failing DAA regimen containing a nucleotide inhibitor (Wyles, 2018b); (Svarovskaia, 2014). This is likely due to the highly conserved catalytic site region that nucleotides bind, making substitutions in this region extremely rare—often referred to as a high barrier to resistance—they are not rare because they do not occur but rather because any such substitution at this site would likely render the virus replication incompetent. Indeed, the specific RAS, S282T, does lead to sofosbuvir resistance but is extremely unfit so it is very rarely detected even after a failed sofosbuvir-containing regimen. When it is found, because of its low fitness level it quickly becomes rare in the population and effectively disappears. Accordingly, this particular RAS is often considered to not be clinically relevant and sofosbuvir may be used for therapy even when it is present. Compounding the clinical impact of NS5A RASs is their ability to maintain high replication competence (aka, relative fitness) in the absence of continued drug pressure, allowing them to remain the dominant viral quasispecies for prolonged periods (years) relative to NS3 protease or NS5B nucleotide polymerase inhibitor RASs, which are typically less fit and tend to disappear over several months, being overcome by more fit wild-type virus species.
The magnitude of the negative impact of both baseline and selected RASs on treatment outcome varies according to regimen (i.e., coadministered drugs); patient factors that impact treatment response (e.g., cirrhosis); and the fold change decrease in potency conferred by the specific RAS(s). Given these considerations, RAS testing alone will not dictate optimal DAA regimen selection. In addition, a drug predicted to suffer a significant loss of potency in the presence of a RAS still may be used in specific clinical settings/regimens.
Terminology, Thresholds of Clinical Relevance, and Assays
Terminology
-
Naming Convention for Hepatitis C Proteins
The hepatitis C genome codes for approximately 5 HCV-specific proteins, which are essential to: 1) form the viral structure (core and envelope proteins); 2) cut the HCV polyprotein; 3) provide enzymatic functions for replication and escape from the innate immune response (NS3/NS4A protease); 4) replicate the HCV RNA (NS5B RNA-dependent RNA polymerase); and 5) bind the HCV replication complex during replication and assembly (NS5A).
-
Polymorphism (Substitution)
A reference (or consensus) nucleotide—and therefore amino acid sequence—has been defined for each HCV genotype. A polymorphism (or substitution) is a difference in an amino acid at a defined position of the HCV protein between a patient’s HCV and the reference HCV protein. Substitution is the preferred terminology among most experts. However, the US Food and Drug Administration currently uses the term polymorphism.
To define a polymorphism, it is necessary to define: the HCV genotype (e.g., genotype 1, 2, 3, etc.) and subtype (e.g., 1a vs 1b); the HCV protein (e.g., NS5A); and the amino acid position (e.g., 93). Polymorphisms are reported as letter-number-letter (e.g., Y93H). The first letter refers to the amino acid typically expected for that position in the reference protein. The number refers to the amino acid position, and the final letter refers to the amino acid that is found in the patient’s HCV isolate. Thus, NS5A Y93H refers to amino acid position 93 of the NS5A protein. The amino acid at this position in the reference strain is Y (i.e., tyrosine) and the amino acid in the tested strain is H (i.e., histidine). For some patients, multiple variants are present and several amino acids may be found at a given position. Thus, it is possible to have a virus with NS5A Y93H/M. Such a patient would have viruses with the amino acids histidine (H) or methionine (M) at position 93 of the NS5A protein.
-
Resistance-Associated Substitutions
A resistance-associated substitution describes any amino acid change from the consensus sequence at a position that has been associated with reduced susceptibility of a virus to 1 or more antiviral drugs. A specific RAS may or may not confer a phenotypic loss of susceptibility to other/multiple antiviral agents.
-
Drug-Class RASs
Drug-class RASs are amino acid substitutions that reduce the susceptibility of a virus to any (and at least 1) member of a drug class or, alternatively, the viral variants with reduced susceptibility that carry these substitutions. Class RASs may or may not confer resistance to a specific drug in that class.
-
Drug-Specific RASs
Drug-specific RASs are amino acid substitutions that reduce the susceptibility of a virus to a specific drug. When assessing the potential clinical impact of RASs on a given regimen, drug-specific RASs should be used. In an HCV-infected population not previously exposed to a DAA drug or class, drug-specific RASs will be found less frequently than class RASs.
Thresholds of Clinical Relevance
HCV resistance to DAAs is a rapidly evolving field with demonstrated clinical impact in specific situations with currently available DAA regimens. Presently, the most clinically significant RASs are in the NS5A position for genotypes 1a and 3.
Data from clinical trials have demonstrated that RASs are commonly, but not always, found at the time of virologic failure. Viruses that are resistant to NS3/4A protease inhibitors seem to be less fit and may disappear from peripheral blood within a few weeks to months, whereas NS5A inhibitor-resistant viruses may persist for years, which could have implications for treatment and retreatment.
In general, drug-specific RASs need to be present in at least 15% of the viruses of a given patient to reduce the likelihood of achieving SVR (Pawlotsky, 2016). Drug-specific RASs that are found at a lower frequency may not convey sufficient resistance to reduce SVR with currently available DAA regimens.
Assays
Methods to detect RASs include population sequencing (aka, Sanger sequencing) and deep sequencing (aka, next generation sequencing [NGS]). Both methods depend on sequencing the HCV RNA, calculating the amino acid sequence, and then inferring the presence of RASs. The methods differ in their sensitivity for detecting RASs. For the purposes of clinical care and decisions regarding which DAA regimen to use, both methods can be considered equivalent if a ≥15% cut point is used for determination of RASs by NGS. Recent studies have shown that NGS at a 1% level of sensitivity often result in the identification of additional RASs that are not associated with clinical failure (Zeuzem, 2017); (Sarrazin, 2016); (Jacobson, 2015b).
-
Genotypic Analysis
-
Population-Based Sequencing (Sanger)
Population sequencing of the HCV coding region of interest may be performed using reverse transcription polymerase chain reaction (PCR) and standard Sanger sequencing of the bulk PCR product. The sensitivity for detection of resistance substitutions varies but is generally 15% to 25%. As a standard, substitutions are reported as differences compared with a genotype-specific, wild-type strain. -
Deep Sequencing Analysis
NGS (deep sequencing approaches) can increase the sensitivity of detection for minor variants. After sequencing HCV coding regions using PCR, a software algorithm is used to process and align sequencing data via a multistep method to identify the substitutions present at a predetermined level. This level, or threshold, can vary but is often set as low as >1% for research purposes. To approximate results obtained by population sequencing, NGS thresholds are often set to ≥10%.
-
Population-Based Sequencing (Sanger)
-
Phenotypic Analysis
Phenotypic analysis involves laboratory techniques whereby the degree of drug resistance conferred by an amino acid change as well as the replicative capacity (fitness) of a particular RAS can be estimated in the presence of a wild-type or consensus strain. These research techniques are not routinely used for clinical practice. To assess the level of resistance, RASs are typically introduced as point mutations into the backbone of an existing standard HCV genome within an existing cell culture/replicon or enzyme-based assay. Isolates harboring these RASs are then challenged by appropriate antiviral agents at increasing concentrations and fold changes—based on EC50 or IC50 and EC90 or IC90 values—are determined for inhibition of replication or enzyme activity, respectively, in comparison to wild-type virus. Comparison of replication levels for variants and wild-type constructs in the absence of drug allows for estimation of fitness.
- Assay Summary Points
- Either population sequencing or deep sequencing can be used to detect the presence of RASs in NS3, NS5A, and NS5B.
- For clinical decisions, population sequencing or deep sequencing with at least 15% prevalence of RASs as the cutoff is recommended. The presence of RASs with <15% prevalence should not be considered clinically significant.
- When assessing the potential clinical effect of RASs, it is important to determine the drug-specific RASs.
Resistance Testing in Clinical Practice
Resistance testing is most important in clinical practice when the results would modify treatment management by impacting the duration of therapy and/or inclusion of ribavirin, or result in selection of alternative therapy. Unfortunately the utility of RAS testing at this time varies by both patient characteristics and DAA regimen.
Approaches to Overcome Resistance
Data for currently approved DAAs provide limited insight on optimal retreatment approaches for patients with a previous DAA therapy failure and high fold change RASs, particularly those in NS5A. Until regimens combining multiple drugs predicted to be active (based on the available resistance profile) are available and adequate phase 2/3 studies in DAA treatment failure populations are accomplished, other aspects of therapy must be optimized in treatment-experienced patients with RASs. In general, optimization involves appropriately characterizing the patient along with use of an extended duration of therapy and the addition of ribavirin (unless an absolute contraindication to ribavirin exists).
Characterizing Patients at Risk
The characteristics that increase the risk of DAA treatment failure are different for each oral regimen. Thus, understanding the population at risk is imperative. Generally, this requires accurate assessment of liver fibrosis and clarification of prior therapy.
Virus
Determination of HCV genotype, subtype, and baseline RASs may be necessary to fully characterize a patient’s risk for therapeutic failure and optimize the treatment approach.
Treatment Duration
The duration of therapy should always be optimized to attain a cure. Although short-duration therapy has been associated with a higher chance of relapse, careful selection of patients for shortened therapy may minimize relapse risk and lead to significant cost savings. In contrast, extension of therapy (often to 24 weeks) in conjunction with the addition of ribavirin has been associated with reasonable SVR rates during retreatment of patients with past DAA therapy failure, even in the presence of significant drug-specific RASs prior to retreatment (Gane, 2017); (Cooper, 2016).
Ribavirin
The addition of ribavirin increases SVR in patient populations with an increased risk for treatment failure (e.g., decompensated cirrhosis). It also improves SVR rates among patients with baseline NS5A RASs and prior DAA treatment failure.
Complementary Therapy
Although data are limited, patients with multiclass RASs can achieve SVR by combining triple or quadruple drug class regimens (see section on retreatment in prior DAA failure). This approach may become less necessary with the approval of standalone dual- or triple-drug regimens composed of second-generation protease and NS5A inhibitors with improved activity against common RASs.
Considerations With Current Antiviral Regimens
Elbasvir/Grazoprevir
Elbasvir/grazoprevir is indicated for treatment-naive and -experienced patients with genotype 1 or 4. The presence of NS3 RASs has no significant impact on SVR12 in patients treated with elbasvir/grazoprevir. The presence of NS5A RASs has no significant impact in genotype 1b infection.
In treatment-naive, genotype 1a patients (with or without cirrhosis) treated with 12 weeks of therapy, the presence of NS3 RASs has no impact (Zeuzem, 2015). In treatment-naive or prior relapse patients treated for 12 weeks with elbasvir/grazoprevir without ribavirin, the presence of high fold change NS5A RASs (at amino acid positions 28, 30, 31, and 93) decreased SVR to 58% (14/24) compared to 98% SVR in those without NS5A RASs. The presence of NS5A RASs had a similar impact on treatment-experienced patients (with or without cirrhosis) who received 12 weeks of elbasvir/grazoprevir without ribavirin (SVR12 29% vs 97%, respectively) (Jacobson, 2015b).
Glecaprevir/Pibrentasvir
In a study of the resistance profiles of glecaprevir and pibrentasvir using cell cultures (Ng, 2017), selection of genotypes 1a, 1b, 2a, 3a, 4a, and 6a replicons for reduced susceptibility to glecaprevir resulted in the emergence of RASs at A156 or D/Q168. The A156 RAS resulted in the greatest reductions (>100-fold) in glecaprevir susceptibility. The D/Q168 RAS had varying effects on glecaprevir susceptibility depending on genotype/subtype and specific amino acid change. The greatest reductions (>30-fold) were observed in genotypes 1a (D168F/Y), 3a (Q168R), and 6a (D168A/G/H/V/Y). These RASs, however, are rarely detected clinically. Pibrentasvir selected no viable colonies in genotype 1b, 2b, 4a, 5a, and 6a. Of the few RASs selected by pibrentasvir, Y93H/N conferred <7-fold resistance.
The presence of baseline RASs had minimal impact on SVR rates with glecaprevir/pibrentasvir in registration trials that predominantly enrolled noncirrhotic patients. In a pooled analysis of NS3/4A protease inhibitor- and NS5A inhibitor-naive patients who received glecaprevir/pibrentasvir in phase 2 and 3 studies (Asselah, 2018b); (Kwo, 2017b); (Forns, 2017); (Foster, 2017); (Zeuzem, 2016), baseline RASs in patients with genotype 1, 2, 4, 5, or 6 infection had no impact on SVR12 (Krishnan, 2018). Among treatment-naive genotype 3 patients without cirrhosis who received glecaprevir/pibrentasvir for 8 weeks, the A30K polymorphism was detected in 10%, of whom 78% achieved SVR12. There are insufficient data to characterize the impact of A30K in genotype 3 patients with cirrhosis or prior treatment experience. All genotype 3 patients with Y93H prior to treatment achieved SVR12.
Ledipasvir/Sofosbuvir
Several comprehensive analyses of genotype 1 patients treated with ledipasvir/sofosbuvir in phase 2 and phase 3 studies have helped clarify the impact of baseline RASs on SVR rates with this regimen (Zeuzem, 2017); (Sarrazin, 2016). In a pooled analysis of patients with genotype 1a or 1b who received ledipasvir/sofosbuvir, 93.5% (316/338) of those with baseline NS5A RASs achieved SVR12 compared to an SVR12 of 98.4% (1,741/1,770) in patients without baseline NS5A RASs (Sarrazin, 2016). In this analysis, the reduction in SVR was driven predominantly by patients with genotype 1a NS5A RASs. The SVR12 rates for genotype 1a patients with and without NS5A RASs were 92.3% and 98.3%, respectively. A slightly lower SVR12 of 90% was observed for genotype 1a patients with NS5A RASs using a 15% deep sequencing cutoff value.
Notably, other factors further delineated populations at risk for relapse in this analysis, including high-level baseline NS5A RASs (>100-fold resistance with Q30H/R, L31M/V, and Y93C/H/N in genotype 1a) and a shorter duration therapy (8 weeks or 12 weeks vs 24 weeks). SVR12 rates were 97.4% to 100% in treatment-experienced patients without NS5A RASs or with RASs with <100-fold resistance treated with ledipasvir/sofosbuvir for 12 weeks or 24 weeks. When RASs with >100-fold resistance were present, however, SVR12 dropped to 64.7% (11/17) with 12 weeks of therapy compared to 100% (6/6) with 24 weeks of therapy. In this small subset of patients, the addition of ribavirin did not appear to offer the same benefit as extension of therapy to 24 weeks in this pooled analysis. SVR12 was 81.8% in those with >100-fold NS5A resistance who received 12 weeks of ledipasvir/sofosbuvir with ribavirin. In contrast, in the SIRIUS trial, all 8 treatment-experienced cirrhotic patients with >100-fold resistance treated for 12 weeks with ledipasvir/sofosbuvir plus ribavirin achieved SVR12.
Sofosbuvir/Velpatasvir
Sofosbuvir/velpatasvir is a pangenotypic therapy indicated for treatment-naive and -experienced patients with or without cirrhosis. In the ASTRAL studies, the presence of NS5A RASs had no impact on SVR12 for patients with genotype 1, 2, 4, 5, or 6 infection treated with 12 weeks of sofosbuvir/velpatasvir (Hézode, 2018). The presence of Y93H in genotype 3 patients decreased the SVR12 to 84% (21/25 patients) compared to 97% (242/249) in those without this RAS (Foster, 2015a). This appeared to be more impactful in patients with cirrhosis and/or prior treatment experience with an interferon-based regimen. Ribavirin was not used in these trials. However, a subsequent trial that randomized patients with genotype 3 and cirrhosis to sofosbuvir/velpatasvir with or without ribavirin demonstrated lower relapse rates in patients receiving ribavirin, but the difference was only relevant in those with baseline Y93H RASs prior to therapy (Esteban, 2018).
Sofosbuvir/Velpatasvir/Voxilaprevir
Sofosbuvir/velpatasvir/voxilaprevir fills an important role as a pangenotypic regimen for patients who have experienced treatment failure with DAA therapy. Although data are limited, the presence of NS3, NS5A, or NS5B RASs prior to treatment did not influence the likelihood of SVR12, and 12 weeks of treatment produced a high SVR12 (96%) in DAA-experienced patients. RAS testing has not been demonstrated to impact SVR rates with sofosbuvir/velpatasvir/voxilaprevir therapy (Sarrazin, 2018); (Bourlière, 2017).
Table 1. Most Common, Clinically Important RASs by DAA, Genotype, and Fold Change
| DAA | Genotype 1a | Genotype 1b | Genotype 3a | |||||
| M28T | Q30R | L31M/V | Y93H/N | L31V/I | Y93H/N | A30K | Y93H | |
| Ledipasvir | 20x | >100x | >100x / >100x | >1000x / >10,000x | >100x | >100x / -- | NA | NA |
| >50x | ||||||||
| Elbasvir | 20x | >100x | >10x | >1000x / >1000x | <10x | >100x / -- | 50x | >100x |
| >100x | ||||||||
| Velpatasvir | <10x | <3x | 20x / 50x | >100x / >1000x | <3x | <3x / -- | 50x | >100x |
| Pibrentasvir | <3x | <3x | <3x | <10x | <3x | <3x | <3x | <3x |
| Color Key: light green = <3-fold change; dark green = <10-fold change; orange = >10- to 100-fold change; pink = >100-fold change | ||||||||
Table 2. Clinically Important RASs by DAA Regimen and Genotype
| DAA Regimen | Genotype | ||
|---|---|---|---|
| 1a | 1b | 3 | |
| Ledipasvir/sofosbuvir |
Q30H/R L31M/V Y93C/H/N |
L31V Y93H |
NA |
| Elbasvir/grazoprevir |
M28A/T Q30H/R L31M/V Y93C/H/N |
Y93H | NA |
| Sofosbuvir/velpatasvir | NA | NA | Y93H |
| Glecaprevir/pibrentasvir | NA | NA | A30K |
Table 3. NS5A RAS Testing Recommendations Prior to Initiation of DAA Treatment Among Genotype 1 Patients by DAA Regimen, Virus Subtype, Prior Treatment Status, and Cirrhosis Status
| DAA Regimen |
1b TNa or TEb |
1a TN |
1a TE No Cirrhosis |
1a TE Cirrhosis |
3 TN Cirrhosis |
3 TE No Cirrhosis |
|---|---|---|---|---|---|---|
| Ledipasvir/sofosbuvir | No | No | Yes | Yes | N/A | N/A |
| Elbasvir/grazoprevir | No | Yes | Yes | Yes | N/A | N/A |
| Sofosbuvir/velpatasvir | No | No | No | No | Yes | Yes |
| Glecaprevir/pibrentasvir | No | No | No | No | No | No |
|
a TN = treatment naive b TE = treatment experienced |
||||||



