

Acute hepatitis C infection is most often asymptomatic and frequently develops into chronic infection. Case reports of acute hepatitis C have increased in the US since 2010 and have most often been associated with parenteral exposures to blood or body fluids (CDC, 2019). Although HCV infection is primarily associated with injection drug use, certain behaviors (eg, unprotected [without a condom] receptive anal intercourse)—primarily among men who have sex with men—are risk factors for transmission (Lockart, 2019); (Price, 2019). The syndemic of opioid use disorder and HCV and HIV transmission contributes to the burden of disease in certain populations (Butt, 2020).
Recommended Testing for Diagnosing Acute HCV Infection |
|
|---|---|
| RECOMMENDED |
RATING |
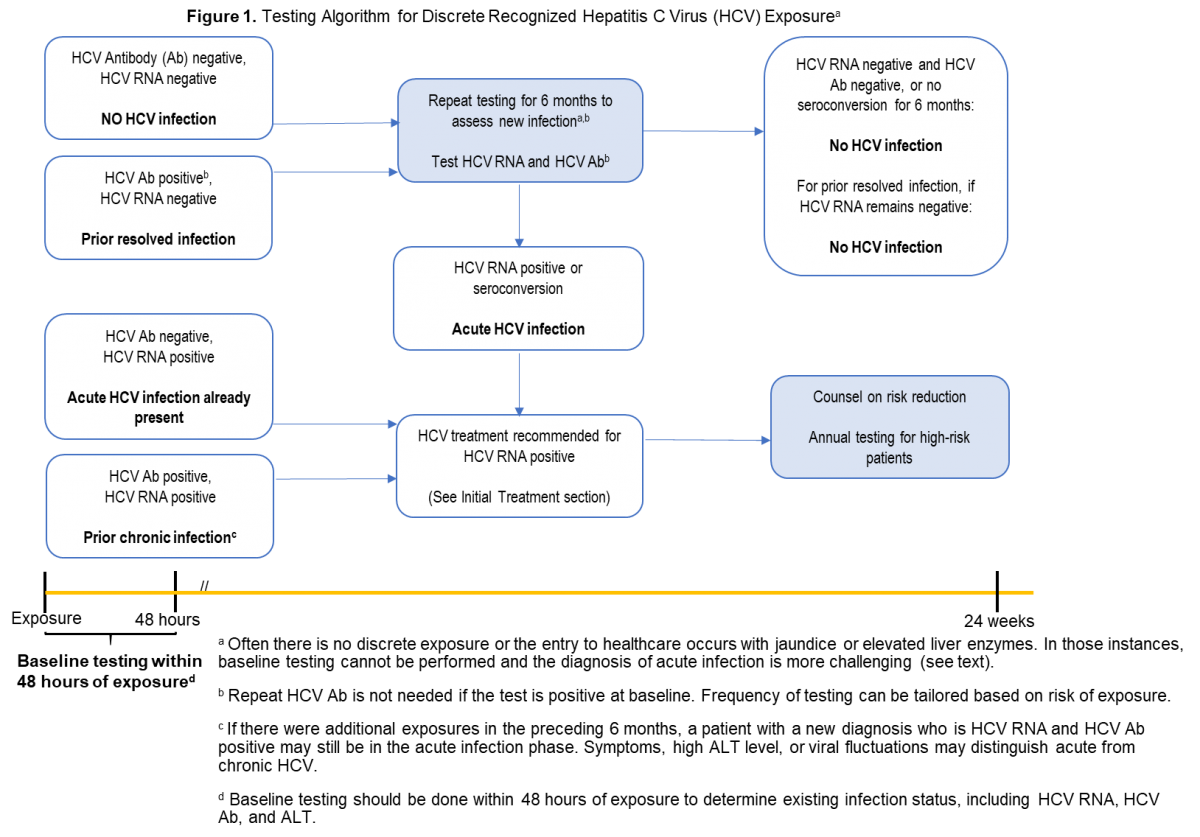
| HCV antibody and HCV RNA testing are recommended when acute HCV infection is suspected due to exposure, clinical presentation, or elevated aminotransferase levels (see Testing Algorithm figure). | I, C |
Recommendations for HCV testing are also found in the Testing and Linkage to Care section.
Diagnosis of acute HCV infection enables estimation of annual incidence rates and transmission patterns, thereby facilitating implementation and assessment of prevention programs. At the individual level, a diagnosis of acute infection expedites linkage to care, counseling regarding high-risk behavior, and timely interventions to reduce virus transmission and liver disease progression (Bruneau, 2014). Some persons involved in high-risk behaviors practice serosorting, defined as using HCV antibody serostatus to determine whether to engage in high-risk behaviors with certain individuals (Smith, 2013). Thus, undiagnosed acutely infected persons may be at greater risk of transmitting HCV to their presumably seronegative contacts than would be expected by chance.
The best laboratory evidence to support a diagnosis of acute HCV infection is a positive HCV RNA test in the setting of a negative HCV antibody test (identification during the seronegative window period) (Cox, 2005), or a positive HCV antibody test after a prior negative HCV antibody test (seroconversion). There are rare instances in which these approaches may be misleading, such as in immunosuppressed individuals with impaired antibody production (Chamot, 1990).
Discrete Exposure
The aforementioned types of clear, laboratory-based documentation of acute HCV infection are most easily achieved when there has been a discrete, known or suspected exposure (eg, after new onset or a change in drug injection practice, a percutaneous needle-stick exposure to an HCV-infected individual, a potentially nonsterile tattoo, or sexual assault). In those instances, baseline HCV antibody and RNA testing should be done within 48 hours of the exposure to document whether there was antecedent HCV infection (see Testing Algorithm figure).
If baseline testing is negative, repeat testing is recommended. Frequency of testing can be tailored based on management objectives (eg, monthly testing to identify and treat acute infection). If baseline HCV antibody testing is positive but RNA testing is negative, repeat HCV RNA and alanine aminotransferase (ALT) testing is recommended to identify an acute reinfection. When baseline HCV antibody and RNA testing are both positive, the person most likely already has chronic HCV infection from prior exposure(s).
No Discrete Exposure
Individuals suspected of having acute HCV infection often do not have a discrete exposure or have no prior baseline testing, making a diagnosis of acute infection more difficult (see Blood Test Interpretation Table). Acute infection should be suspected if there is a new rise in the ALT level without an alternate cause (Blackard, 2008); (Kim, 2013). Acute infection should also be suspected when there are low (especially <104 IU/mL) or fluctuating (>1 log10 IU/mL) HCV RNA values, or spontaneous clearance. These patterns do not commonly occur outside of the first 6 months after HCV infection (McGovern, 2009). In those with a high index of suspicion for HCV exposure (eg, recently relapsed injection drug use, other high-risk exposure), an HCV PCR should be repeated, if negative.
Patients suspected of having acute HCV infection should also have a laboratory evaluation to exclude other or coexisting causes of acute hepatitis (eg, hepatitis A virus, hepatitis B virus, hepatitis delta virus if chronically infected with hepatitis B, and autoimmune hepatitis) (Kushner, 2015). In patients with sexual acquisition of acute HCV, evaluation for concurrent genital ulcerative disease and proctitis is recommended (Todesco, 2019); (Goldenberg, 2017). Patients should also have HIV testing.
Table. Interpretation of Blood Tests for Diagnosis of Acute HCV Infection
| TEST | INTERPRETATION FOR DIAGNOSIS OF ACUTE HCV |
|---|---|
| HCV Antibody |
|
| HCV RNA |
|
| ALT |
|
There are no data on the efficacy or cost-effectiveness of antiviral therapy for pre-exposure or post-exposure prophylaxis of HCV infection.
Patients with acute HCV infection should be treated upon initial diagnosis without awaiting spontaneous resolution, using a “test and treat” strategy and according to the simplified approach, if eligible. Real-world data have demonstrated a reduction in HCV viremia prevalence and incidence with unrestricted access to HCV therapy (Boerekamps, 2018). In addition, mathematical modeling suggests that DAA treatment scale-up, especially among those at highest risk of transmission, can reduce HCV incidence and prevalence (Martin, 2013); (Martin, 2016). Moreover, delay introduced by waiting for spontaneous clearance may be associated with loss to follow up.
Individuals with acute HCV should be counseled to reduce behaviors that could result in virus transmission, such as sharing injection equipment and engaging in high-risk sexual practices. Because the risk of transmission of other bloodborne, sexually transmitted infections (eg, HIV and HBV) is higher in the acute infection phase, some experts counsel patients with acute HCV to consider using barrier precautions, even in a stable monogamous relationship (see Testing and Linkage to Care). For individuals with acute HCV infection who have a history of recent injection drug use, referral to harm reduction services and an addiction medicine specialist is recommended when appropriate (Litwin, 2009); (Strathdee, 2005).
Patients with acute hepatitis C are often asymptomatic or have nonspecific symptoms (eg, fatigue, anorexia, mild or moderate abdominal pain, low-grade fever, nausea, and/or vomiting) that frequently are not recognized as being associated with acute HCV infection. A small proportion (<25%) of patients with acute HCV develop jaundice. Patients diagnosed with acute HCV should initially be monitored with hepatic panels (ALT, aspartate aminotransferase [AST], bilirubin, and international normalized ratio [INR] in the setting of an increasing bilirubin level) at 2- to 4-week intervals (Blackard, 2008). With treatment, a rapid improvement of laboratory parameters is expected.
There is no need to alter concomitant medications that are metabolized by hepatic enzymes unless there is concern for developing acute liver failure (eg, increasing bilirubin level and INR). Acetaminophen and alcohol consumption should be avoided during acute HCV infection (Proeschold-Bell, 2012); (Dieperink, 2010); (Whitlock, 2004).
Hospitalization is rarely indicated unless nausea and vomiting are severe. Although acute liver failure is very rare (<1%), it represents a serious and life-threatening complication of acute HCV infection. Patients with an INR >1.5 and those who exhibit any signs of acute liver failure (eg, hepatic encephalopathy) should be referred to a liver transplant center immediately. Use of HCV antiviral regimens in acute liver failure should be managed by a clinician experienced in HCV treatment, ideally in consultation with a liver transplant specialist.
HCV infection spontaneously clears in 20% to 50% of patients (Kamal, 2008). Clearance of acute HCV infection occurs within 6 months of the estimated time of infection (median, 16.5 weeks) in at least 2/3 of patients who spontaneously clear HCV. Only 11% of those who remain viremic at 6 months will spontaneously clear the infection at a later time (Grebely, 2014). Patients who have spontaneously cleared should not be treated with antiviral therapy. However, they should be counseled about the possibility of reinfection and tested routinely for this development if risk behaviors are ongoing (see Testing and Linkage to Care). Of note, transient suppression of viremia can occur in those with acute HCV infection, even among those who progress to chronic infection. Thus, a single undetectable HCV RNA test result is insufficient to declare spontaneous clearance (see Testing and Linkage to Care) (Villano, 1999); (Mosley, 2008).
Predictors of spontaneous clearance include jaundice, elevated ALT level, hepatitis B virus surface antigen (HBsAg) positivity, female sex, younger age, genotype 1 infection, and host genetic polymorphisms, most notably those near the IL28B gene (Kamal, 2008); (Mosley, 2008).
A number of studies have evaluated DAA treatment of acute HCV infection. Small single-arm, uncontrolled studies have evaluated 6 or 8 weeks of ledipasvir/sofosbuvir. One such study demonstrated 100% SVR with 8 weeks of ledipasvir/sofosbuvir among 27 men with acute HCV and HIV-coinfection (Naggie, 2019). Investigators conducting another study evaluated 6 weeks of ledipasvir/sofosbuvir in a similar cohort (25/26 with HIV coinfection). Among participants with genotype 1 infection, 79% (15/19) achieved SVR12; 71% (5/7) of those with genotype 4 infection achieved SVR12 with this shortened regimen. Among the 6 individuals whose treatment did not lead to SVR12, there were 3 relapses (all had baseline HCV RNA levels >7 log10 IU/mL). Three participants achieved SVR4 but were lost to follow-up (Rockstroh, 2017b). A phase 2 study followed a similar treatment protocol (ie, 6 weeks of ledipasvir/sofosbuvir) among 20 individuals with genotype 1 HCV monoinfection, all of whom achieved SVR12 (Deterding, 2017).
An open-label, single-arm, multicenter pilot study evaluated the efficacy of 6 weeks of the pangenotypic regimen glecaprevir/pibrentasvir among persons with acute/recent HCV infection (ie, duration of infection <12 months). SVR12 was 90% (27/30); a single virological failure occurred in a man with genotype 1a, HIV coinfection, and a viral load of 7.7 log10 IU/mL. This patient was successfully retreated (Martinello, 2020).
In the only randomized trial to date, investigators compared 6 vs 12 weeks of sofosbuvir/velpatasvir in the international REACT trial of acute/recent infection. The study was stopped early due to inferiority of the shortened (ie, 6 week) arm. In the 6-week arm, 81.7% (76/93) (on ITT) and 89.4% (76/85) (on mITT) of participants achieved SVR with 6 relapses and 8 nonvirologic failures. In the 12-week group, 90.5% (86/95) on ITT and 97.7% (86/88) (on mITT) achieved SVR with no virologic failures (3 participants were lost to follow-up). There were no clear predictors of relapse aside from shorter treatment duration (Matthews, 2021).
To date, there are insufficient data to support a particular regimen or treatment duration outside of a clinical trial. Until more definitive data are available, treatment as described for chronic hepatitis C is recommended (see Initial Treatment of HCV Infection). Pangenotypic regimens are recommended if HCV genotyping is unavailable or if concern of exposure to more than 1 genotype exists. Using the same regimens to treat acute/recent HCV as for chronic HCV infection also simplifies management, as defining acute HCV may be clinically challenging.